Identification of potent biparatopic antibodies targeting FGFR2 fusion-driven cholangiocarcinoma
- PMID: 40014401
- PMCID: PMC11996885
- DOI: 10.1172/JCI182417
Identification of potent biparatopic antibodies targeting FGFR2 fusion-driven cholangiocarcinoma
Abstract
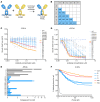
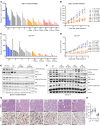
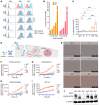
Translocations involving FGFR2 gene fusions are common in cholangiocarcinoma and predict response to FGFR kinase inhibitors. However, response rates and durability are limited due to the emergence of resistance, typically involving FGFR2 kinase domain mutations, and to suboptimal dosing, relating to drug adverse effects. Here, we develop biparatopic antibodies targeting the FGFR2 extracellular domain (ECD) as candidate therapeutics. Biparatopic antibodies can overcome drawbacks of bivalent monospecific antibodies, which often show poor inhibitory or even agonist activity against oncogenic receptors. We show that oncogenic transformation by FGFR2 fusions requires an intact ECD. Moreover, by systematically generating biparatopic antibodies targeting distinct epitope pairs in FGFR2 ECD, we identified antibodies that effectively block signaling and malignant growth driven by FGFR2 fusions. Importantly, these antibodies demonstrate efficacy in vivo, synergy with FGFR inhibitors, and activity against FGFR2 fusions harboring kinase domain mutations. Thus, we believe that biparatopic antibodies may serve as an innovative treatment option for patients with FGFR2-altered cholangiocarcinoma.
Keywords: Drug therapy; Liver cancer; Oncology; Signal transduction; Therapeutics.
Conflict of interest statement
Figures




Update of
-
Identification of potent biparatopic antibodies targeting FGFR2 fusion driven cholangiocarcinoma.bioRxiv [Preprint]. 2024 Sep 18:2024.09.16.613045. doi: 10.1101/2024.09.16.613045. bioRxiv. 2024. Update in: J Clin Invest. 2025 Feb 27;135(8):e182417. doi: 10.1172/JCI182417. PMID: 39345400 Free PMC article. Updated. Preprint.
References
-
- Goyal L, et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9(8):1064–1079. doi: 10.1158/2159-8290.CD-19-0182. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

