Megakaryocytes transfer mitochondria to bone marrow mesenchymal stromal cells to lower platelet activation
- PMID: 40014405
- PMCID: PMC11996913
- DOI: 10.1172/JCI189801
Megakaryocytes transfer mitochondria to bone marrow mesenchymal stromal cells to lower platelet activation
Abstract
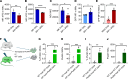
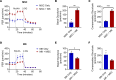
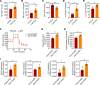
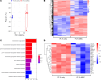
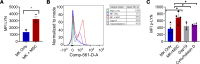
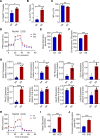
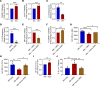
Newly produced platelets acquire a low activation state, but whether the megakaryocyte plays a role in this outcome has not been fully uncovered. Mesenchymal stem cells (MSCs) were previously shown to promote platelet production and lower platelet activation. We found that healthy megakaryocytes transfer mitochondria to MSCs, which is mediated by connexin 43 (Cx43) gap junctions on MSCs and leads to platelets at a low energetic state with increased LYN activation, characteristic of resting platelets with increased LYN activation, characteristic of resting platelets. On the contrary, MSCs have a limited ability to transfer mitochondria to megakaryocytes. Sickle cell disease (SCD) is characterized by hemolytic anemia and results in heightened platelet activation, contributing to numerous disease complications. Platelets in SCD mice and human samples had a heightened energetic state with increased glycolysis. MSC exposure to heme in SCD led to decreased Cx43 expression and a reduced ability to uptake mitochondria from megakaryocytes. This prevented LYN activation in platelets and contributed to increased platelet activation at steady state. Altogether, our findings demonstrate an effect of hemolysis in the microenvironment leading to increased platelet activation in SCD. These findings have the potential to inspire new therapeutic targets to relieve thrombosis-related complications of SCD and other hemolytic conditions.
Keywords: Bone marrow; Hematology; Mouse stem cells; Platelets; Stem cells.
Conflict of interest statement
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

