Efficacy of Pharmacological Interventions in Milder Depression: A Systematic Review and Meta-Analysis
- PMID: 40014460
- PMCID: PMC11867163
- DOI: 10.1002/npr2.70008
Efficacy of Pharmacological Interventions in Milder Depression: A Systematic Review and Meta-Analysis
Abstract
Background: Mild depression lacks a consistent definition across diagnostic criteria and rating scales, posing challenges to standardizing treatment strategies. International guidelines predominantly recommend psychotherapy as the first-line treatment for mild depression, while the use of antidepressants remains contentious. Supplements such as omega-3 fatty acids, St. John's Wort, and magnesium have garnered attention as alternative therapeutic options for depression. This systematic review aims to assess the efficacy of pharmacological interventions, including supplements, in the treatment of mild depression.
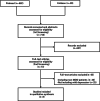
Methods: Comprehensive searches were performed in PubMed and Embase through November 2024 to identify randomized controlled trials (RCTs) investigating pharmacotherapy or supplements for mild depression diagnosed using standardized criteria. Eligible studies underwent screening and risk of bias assessment utilizing the ROB2 tool. Data on remission rates, symptom improvement, dropout rates, and adverse events were extracted, with meta-analyses conducted where applicable.
Results: Eight RCTs comprising 1049 participants met inclusion criteria. Among the agents studied, St. John's Wort was analyzed in two trials, both comparing it to fluoxetine. A meta-analysis found no significant difference in response rates between the two treatments (risk ratio [RR] = 0.96, 95% CI: 0.78-1.18) or dropout rates (RR = 1.08, 95% CI: 0.62-1.88). For other agents, single studies evaluated their effects. Eicosapentaenoic acid and Rhodiola rosea demonstrated significant improvements in depressive symptoms compared to placebo. In non-blinded trials, magnesium chloride showed efficacy in alleviating depressive symptoms. Other interventions, such as lavender, lemon balm, and transcranial electroacupuncture stimulation, were as effective as antidepressants. Conversely, S-adenosylmethionine did not produce significant improvements relative to placebo.
Conclusion: This review demonstrates that certain supplements, such as eicosapentaenoic acids and Rhodiola rosea, are therapeutic options for mild depression. However, no RCTs compared antidepressants directly to placebo for mild depression. The paucity of high-quality RCTs exclusively targeting mild depression limits definitive conclusions, warranting further rigorous research.
Keywords: St. John's Wort; mild depression; omega‐3 fatty acids; pharmacotherapy; supplements.
© 2025 The Author(s). Neuropsychopharmacology Reports published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Society of Neuropsychopharmacology.
Conflict of interest statement
MU has received speaker honoraria from Sumitomo Pharma and Janssen Pharmaceutical over the last 3 years. HS received grants from the Japan Society for the Promotion of Science, the Japan Research Foundation Clinical Pharmacology, and the Takeda Science Foundation, and an honorarium from Viatris, Eisai, Takeda Pharmaceutical, Otsuka Pharmaceutical, Meiji Seika Pharma, Shionogi Pharma, Yoshitomiyakuhin, Sumitomo Pharma, Kyowa Pharmaceutical, MSD, and Lundbeck Japan. HS is an Editorial Board member of Neuropsychopharmacology Reports and a corresponding author of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication. FU has received grants from the Nakatani Foundation, the Canadian Institutes of Health Research (CIHR), and the Brain & Behavior Research Foundation (BBRF); manuscript fees from Dainippon Sumitomo Pharma; and consultant fees from WCG Clinical and Uchiyama Underwriting within the past three years. TM has nothing to declare. T. Tada has received speaker honoraria from Dainippon Sumitomo Pharma and Otsuka Pharmaceutical. TU has nothing to declare. YM received an honorarium from Sumitomo Pharma, Janssen Pharmaceutical, and Meiji Seika Pharma. MM received an honorarium from Sumitomo Pharma, Yoshitomiyakuhin. MT has nothing to declare. HB received grant funding from the Japan Society for the Promotion of Science and speaker's honoraria from Otsuka Pharmaceutical, Sumitomo Dainippon Pharma, Viatris, MSD, Meiji Seika Pharma, Eli Lilly, Yoshitomi Yakuhin, Janssen Pharmaceutical, Kyowa Pharmaceutical, Mitsubishi Tanabe Pharma, Pfizer, Esai, Takeda Pharmaceutical, Lundbeck Japan, Mochida, Sawai, Kowa, EA Pharma, and Mylan EPD. MK has received grant funding from AMED, the Japanese Ministry of Health, Labour and Welfare, the Japan Society for the Promotion of Science, SENSHIN Medical Research Foundation, the Japan Research Foundation for Clinical Pharmacology, and the Japanese Society of Clinical Neuropsychopharmacology, and consulting fees from Sumitomo Pharma Co. Ltd., Shionogi & Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Lundbeck Japan K.K., Boehringer Ingelheim Co. Ltd., and Takeda Pharmaceutical Co. Ltd.; payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Sumitomo Pharma Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Meiji Seika Pharma Co. Ltd., Shionogi & Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co. Ltd., Lundbeck Japan K.K., Viatris Inc., Eisai Co. Ltd., and Kyowa Pharmaceutical Industry Co. Ltd. T. Tsuboi received grants from the Japan Society for the Promotion of Science and an honorarium from Takeda Pharmaceutical, Otsuka Pharmaceutical, Meiji Seika Pharma, Shionogi Pharma, Yoshitomiyakuhin, Sumitomo Pharma, Kyowa Pharmaceutical, MSD, Nippon Boehringer Ingelheim, Mylan EPD, Mitsubishi Tanabe Pharma, Viatris, Mochida Pharmaceutical, Janssen Pharmaceutical, TEIJIN PHARMA, and Lundbeck Japan. KW has received consultant fees from Boehringer Ingelheim, Daiichi Sankyo, Eisai, Lundbeck Japan, Luye Pharma, Mitsubishi Tanabe Pharma, Nippon Chemiphar, Ono Pharmaceutical, Otsuka Pharmaceutical, Sumitomo Pharma, and Takeda Pharmaceutical, received grant funding from AMED, the Japan Society for the Promotion of Science, and speaker honoraria from Boehringer Ingelheim, Eisai, Janssen Pharmaceutical, Kyowa Pharmaceutical, Lundbeck Japan, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Otsuka Pharmaceutical, Shionogi, Sumitomo Pharma, Takeda Pharmaceutical, and Viatris.
Figures



References
-
- World Health Organization , “International Classification of Diseases 11th Revision,” accessed September 29, 2024, https://icd.who.int/en.
-
- American Psychiatric Association , “Diagnostic & Statistical Manual of Mental Disorders, Fifth Edition, Text Revision,” (2022).
-
- Lam R. W., Kennedy S. H., Adams C., et al., “Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: Réseau Canadien Pour les Traitements de L'humeur et de L'Anxiété (CANMAT) 2023: Mise à Jour des Lignes Directrices Cliniques Pour la Prise en Charge du Trouble Dépressif Majeur Chez Les Adultes,” Canadian Journal of Psychiatry 69, no. 9 (2024): 7067437241245384, 10.1177/07067437241245384. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

