Aligning cellular and molecular components in age-dependent tertiary lymphoid tissues of kidney and liver
- PMID: 40014629
- PMCID: PMC11867392
- DOI: 10.1371/journal.pone.0311193
Aligning cellular and molecular components in age-dependent tertiary lymphoid tissues of kidney and liver
Abstract
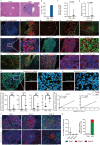
Tertiary lymphoid tissues (TLTs) are ectopic lymphoid structures induced by multiple stimuli, including infection and tissue injuries; however, their clinical relevance in disease progression has remained unclear. We demonstrated previously that TLTs develop in mouse and human kidneys with aging and can be a potential marker of kidney injury and prognosis, and therapeutic targets. In addition, we found that two types of unique lymphocytes that emerge with aging, senescence-associated T cells and age-associated B cells, are essential for TLT formation in the kidney. Although TLTs develop with aging in other organs as well, their cellular and molecular components, and clinical significance remain unclear. In the present study, we found that TLTs developed in the liver with aging, and that their cellular and molecular components were similar to those in the kidneys. Notably, senescence-associated T cells and age-associated B cells were also present in hepatic TLTs. Furthermore, analysis of publicly available data on human liver biopsy transcriptomes revealed that the expression of TLT-related genes was elevated in the liver biopsy samples from hepatitis C virus (HCV)-infected patients compared with those without HCV infection and was associated with liver injury and fibrosis. Therefore, we analyzed liver biopsy samples from 47 HCV patients and found that TLTs were present in 87.2% of cases and that the numbers and stages of TLTs were higher in aged patients and cellular and molecular components of TLTs in humans were similar to those in mice. Our findings suggesting that age-dependent TLT formation is a systemic phenomenon across the tissues and aging is also a predisposing factor for TLT formation across organs.
Copyright: © 2025 Toriu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
YS was employed by the TMK project, a collaboration project between Kyoto University and Mitsubishi Tanabe Pharma. MY has received research grants from Mitsubishi Tanabe Pharma and Boehringer Ingelheim. MH was employed by the Immunosenescence project, which was a collaboration project between Kyoto University and Ono Pharmaceutical Co. Ltd. Other authors report no conflicts of interest.
Figures