Trends in Cervical Precancers Identified Through Population-Based Surveillance - Human Papillomavirus Vaccine Impact Monitoring Project, Five Sites, United States, 2008-2022
- PMID: 40014651
- PMCID: PMC11867585
- DOI: 10.15585/mmwr.mm7406a4
Trends in Cervical Precancers Identified Through Population-Based Surveillance - Human Papillomavirus Vaccine Impact Monitoring Project, Five Sites, United States, 2008-2022
Abstract
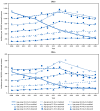
In 2006, human papillomavirus (HPV) vaccine was first recommended in the United States to prevent cancers and other diseases caused by HPV; vaccination coverage increased steadily through 2021, and increasing numbers of young women had received HPV vaccine as children or adolescents. Since 2008, CDC has monitored incidence of precancerous lesions (cervical intraepithelial neoplasia [CIN] grades 2-3 and adenocarcinoma in situ [AIS], collectively CIN2+), which are detected through cervical cancer screening and can be used as an intermediate outcome for monitoring vaccination impact, via the five-site Human Papillomavirus Vaccine Impact Monitoring Project. This analysis describes trends in incidence of CIN2+ and CIN3+ (i.e., CIN grade 3 and AIS) lesions during 2008-2022. Among women aged 20-24 years who were screened for cervical cancer, rates during 2008-2022 decreased for CIN2+ by 79%, and for CIN3+ by 80%. In the same period, CIN3+ rates among screened women aged 25-29 years decreased by 37%. These data are consistent with considerable impact of HPV vaccination for preventing cervical precancers among women in the age groups most likely to have been vaccinated, and support existing recommendations to vaccinate children at the routinely recommended ages as a cancer prevention measure.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Jessica L. Castilho reports institutional support from the National Institutes of Health and receipt of equipment, materials, drugs, medical writing, gifts or other services from Copan Diagnostics. Linda M. Niccolai reports institutional support from the National Institutes of Health, receipt of consulting fees and payment for expert testimony from Merck, and payment for participation on a GSK advisory board. Emily L. Delikat reports support from the Association of Immunization Managers to attend Vaccine Access Collaborative meetings and compensation to serve as Director of Tennessee Families for Vaccines, a part of the SAFE Communities Coalition. No other potential conflicts of interest were disclosed.
Figures
References
-
- CDC. Cancer: cancers linked with HPV each year. Atlanta, GA: US Department of Health and Human Services, CDC; 2024. Accessed May 29, 2024. https://www.cdc.gov/cancer/hpv/cases.html

