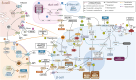
Fig. 4
T1D targets for therapeutic interventions in immune cells. Immune cells: (1) Anti-thymocyte globulin (ATG) induces broad nonspecific immunosuppression that is primarily mediated through the recognition of a series of antigens expressed on human lymphohematopoietic cells, such as CD2, CD3, CD4, and CD8 expressed on T-cells, CD19 and CD20 expressed on B cells, and CD11b, CD80, and CD86 expressed on antigen-presenting cells (APCs). ATG achieves immunosuppression by eliminating lymphocytes in the recirculating pool through complement-mediated intravascular lysis, apoptosis, and antigen-dependent cell-mediated cytotoxicity. Low-dose ATG reduced HbA1c 2 years after therapy in recent-onset T1D patients. (2) Anti-CD3 monoclonal antibody (teplizumab) blockade of CD3 receptors in T-cells induces a state of anergy in certain T-cell populations, making them unresponsive to specific stimuli, and promoting regulatory T-cell functions. In recent-onset T1D patients, teplizumab was observed to preserve β-cell function and slow down C-peptide degradation. Costimulatory signal blockade in T-cells: (3) Abatacept is a CTLA-4/Fc fusion protein that prevents T-cell CD28 interaction with its CD80/86 ligand on APCs, thereby limiting immune system activation. Abatacept modified progression of T1D by significantly impacting CD4+ cell subsets, thereby delaying the decline of C-peptide and improving HbA1c in recent-onset T1D patients. (4) Alefacept is an anti-CD2 fusion protein that has a dual function, as it triggers PCD of activated memory T-cells and inhibits the interaction between leukocyte-function-associated antigen (LFA-3) and CD2, effectively preventing costimulatory signaling for the activation and proliferation of T-cells. In recent-onset T1D patients, 2 12-week courses of alefacept delayed C-peptide decline and depleted CD4+, CD8+ T-cells, and effector memory T-cells for over a year after cessation of therapy. (5) High expression of membrane-bound dipeptidyl peptidase-4 (mDPP-4) is associated with the differentiation of T lymphocytes into Th1 (IL-2, interferon gamma) and Th17 (IL-6, IL-17, and IL-22) cells and upon activation of B cells. DPP-4 inhibitors (DPP-4i) disrupt the mDPP4-caveolin-1 nuclear factor kappa B (NFκB) activation pathway, which leads to a decrease in the expression of CD86 on antigen-presenting cells (APCs) and other monocytes. This limits the interaction between CD86 and CD28 on T-cells, resulting in a reduction in the proliferation and activation of antigen-specific T-cells. Additionally, DPP-4 inhibition prevents binding of mDPP-4 with adenosine deaminase (ADA) that would otherwise lead to the formation of a costimulatory CD3 signaling complex in CD4+ T-cells initiating CARMA-1 signaling. Furthermore, DPP-4 inhibitors prevent activation of Th1 cells, thereby leading to decreased secretion of proinflammatory cytokines: IL-1β, interferon gamma, TNF-α, and IL-2 from Th1 cells. GLP-1: (6) Activated T-cells express a higher number of functional glucagon-like peptide-1 receptors (GLP-1R) in human CD4+ T-cells and GLP-1R activation by GLP-1 receptor agonists (GLP-1RA) in Treg cells leads to increased IL-10 expression and enhanced cellular inhibitory function. T regulatory cells (Treg cells): (7) DPP-4 inhibition in CD4+ T-cells is found to promote the function of Treg cells and the production of the immunosuppressive cytokine TGF-β. Immunosuppressive functions of Treg cells are facilitated through CTLA-4 ligand binding to CD80/86 on APCs, expression of immunosuppressive cytokines: IL-10, TGF-beta and CD39-induced hydrolyzation of ATP to adenosine (ADO). Treg-derived ADO is a hydrolysis product of extracellular ATP cleaved in tandem by 2 Treg-associated ectonucleotidases, CD39 and CD73. (8) Most naïve CD8+ T-cells and a small number of mature CD4+ and Treg cells express CD73 on their surface. Enzyme-active CD73 is released from the CD8+ T-cell membrane upon activation, allowing Treg cell-driven ATP hydrolysis to occur, leading to adenosine formation. In immune cells, adenosine binds to the A2A receptor (A2aR), leading to the elevation of cAMP and interfering with the functions of activated T-cells and APCs, inhibiting their proliferation and cytokine production. IL-21: (9) IL-21 is produced primarily by CD4+ T-cells and is required for both Th17 cell differentiation and the generation of T follicular helper (Tfh) cells. IL-21 is the most prominent cytokine for the activation and differentiation of human B cells. IL-21 induces the differentiation of human naive and memory B cells into antibody-secreting plasma cells. Other cytokines, such as IL-4, greatly inhibit IL-21-driven plasma cell differentiation. IL-21 also directly regulates B-cell proliferation and apoptosis and can promote immunoglobulin production and isotype class switching. In addition, IL-21 signaling enhances the cytotoxicity of CD8+ T-cells and natural killer (NK) cells. A combination treatment of anti-IL-21 and GLP-1RA (liraglutide) preserved β-cell function in recent-onset T1D patients. This was demonstrated by a reduction in the concentration of C-peptide, as measured during a mixed-meal tolerance test (MMTT), from the baseline measurement to week 54 of treatment. B cells: (10) Anti-CD20 mAb (rituximab) effectively depletes mature B cells by various mechanisms inducing cell death, including DNA fragmentation, complement-dependent cytotoxicity (CDC), and programmed cell death (PCD). CD20 is reported to regulate B-cell differentiation and growth as well as adjusting Ca2+ transport. Notably, CD20 is detectable in pre-B cells to mature B cells but is absent in antigen-producing plasma cells. GABA: (11) Gamma-aminobutyric acid (GABA) has broad immune-modulating properties. It controls the release of cytokines from CD4+ T-cells and anti-CD3-stimulated peripheral blood mononuclear cells (PBMCs). Importantly, GABA suppresses the release of 47 cytokines in PBMCs from T1D patients and regulates pro- and anti-inflammatory cytokine production in a concentration-dependent manner. Engagement of the GABAA receptor (GABAAR) induces depolarization of the membrane potential, leading to inhibition of T-cell responses. B cells secrete GABA, which then inhibits inflammatory cytokine production in CD8+ T-cells and stimulates monocyte differentiation into IL-10-secreting immunosuppressive cells. Calcium blockade: (12) Lymphocyte calcium channel blockade may be an effective immunosuppressive strategy. Verapamil had a significant impact on T-cell activation by strongly inhibiting the expression of CD25 (which is typically present in Tregs), CD40L, and CD69. This inhibition is likely due to the failure of Ca2+-dependent transcription factors to activate gene transcription. Nuclear factor of activated T-cells (NFAT) is triggered by Ca2+, which also triggers the production of other transcription factors, including IRF4 and HIF-1α, that control the metabolic switch, cell cycle progression, and proliferation of activated human T-cells. Verapamil partially preserves β-cell function, as shown by C-peptide secretion in children and adolescents with recent-onset T1D. Proton pump inhibitors: (13) In immune cells, proton pump inhibitors (PPIs) suppress T-cell responses by decreasing the expression of the T-cell receptor (TCR)-activated membrane zinc transporter Zip8, thereby lowering the cytoplasm-free zinc (Zn) concentration. PPI-induced decrease in Zip8 expression increases transcription factor CREMα, which dramatically downregulates IL-2 production, while decreases in the transcription factor pCREB downregulates production of interferon gamma in lymphocytes. In monocytes, PPIs were found to reduce the production of several inflammatory cytokines: TNF-α, IL-1β, IL-6, and NFκB. Moreover, PPIs inhibit the activation of neutrophils and monocytes and deplete intracellular and extracellular neutrophil reactive oxygen species (ROS) and nitric oxide (NO). PPI with DPP-4i in recent-onset T1D patients did not achieve C-peptide preservation, but due to high safety, PPI and DPP-4i have been suggested to be used in combination with other drugs. Preliminary results show that a combination of GABA, DPP-4i, and PPI as an adjunct to insulin therapy improves glycemic control in patients with T1D and elevates C-peptide levels in recent-onset T1D patients. Beta-cells: Cytokine blockade. (A) During the progression of T1D macrophages and T-cells invade the islets and secrete proinflammatory cytokines. The combination of TNF-α and interferon gamma synergistically induces β-cell apoptosis through activation of JNK/SAPK, resulting in the production of reactive oxidative species (ROS) and loss of mitochondrial transmembrane potential (ΔΨm). Proinflammatory cytokine blockade may act to prevent deleterious effects on β-cell survival and function in the islet microenvironment. Anti-inflammation and cytokine-modifying therapies showed varying degrees of effectiveness as TNF-α monoclonal antibodies (eg, Golimumab and Etanercept) postponed C-peptide loss in patients with recent-onset T1D. Canakinumab binds human IL-1β with high affinity and neutralizes its biological activity while Anakinra is an IL-1 receptor antagonist. Due to high safety, but insufficient efficacy in recent-onset T1D patients, canakinumab and anakinra have been suggested for IL-1β blockade as part of combination therapies. Verapamil + IGF-1: (B) Verapamil downregulates Ca2+ influx and thereby disrupts the formation of thioredoxin-interacting protein (TxNIP), reduces β-cell expression of IGF-binding protein 3 (IGFBP3) and thereby elevates IGF-1 induced signaling via increased IGF-1. (C) Stimulation of IGF-1R initiates PI3K/Akt signaling, which enables the activation of mTORC1. Upon activation mTORC1 phosphorylates the 4EBP1 protein, promoting cell growth, and the p70 ribosomal protein S6 kinase (S6K1), resulting in enhanced ribosomal biogenesis, mitochondrial biogenesis, and oxygen consumption. PI3K/Akt signaling also promotes β-cell-, but inhibits α-cell-related gene expression, as well as inhibiting β-cell apoptosis in the context of inflammatory cytokines and oxidative stress. Importantly, insulin receptor (IR) and IGF-1R are highly homologous and share PI3K/Akt and Ras/MAPK signaling pathways IR largely controls metabolism, whereas IGF-1R controls growth. (D) IGFBP-3 is a negative regulator of β-cell mass independent of IGF-1, which is a positive regulator. IGFBP-3 is a binding ligand to the death receptor TMEM219, which is widely expressed in islet β-cells. Bound TMEM219 triggers Caspase-8-mediated apoptosis of β-cells. (E) A 26-week course of a small tyrosine kinase inhibitor, imatinib mesylate (Gleevec, STI571) preserved β-cell function at 12 months in adults with recent-onset T1D. Imatinib acts as a β-cell protective drug, as it reduces ER stress and consequent β-cell apoptosis by inhibiting ABL kinase binding and hyperactivation of ER transmembrane kinase’s endoribonuclease (IRE1α RNase).