Global, regional, and national burden of epilepsy, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021
- PMID: 40015291
- PMCID: PMC11876103
- DOI: 10.1016/S2468-2667(24)00302-5
Global, regional, and national burden of epilepsy, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021
Abstract
Background: Epilepsy is one of the most common serious neurological disorders and affects individuals of all ages across the globe. The aim of this study is to provide estimates of the epilepsy burden on the global, regional, and national levels for 1990-2021.
Methods: Using well established Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) methodology, we quantified the prevalence of active idiopathic (epilepsy of genetic or unknown origin) and secondary epilepsy (epilepsy due to an underlying abnormality of the brain structure or chemistry), as well as incidence, death, and disability-adjusted life-years (DALYs) by age, sex, and location (globally, 21 GBD regions and seven super-regions, World Bank country income levels, Socio-demographic Index [SDI], and 204 countries) and their trends from 1990 to 2021. Vital registrations and verbal autopsies provided information about deaths, and data on the prevalence and severity of epilepsy, largely came from population representative surveys. All estimates were calculated with 95% uncertainty intervals (UIs).
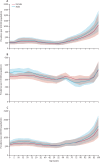
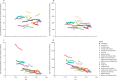
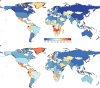
Findings: In 2021, there were 51·7 million (95% UI 44·9-58·9) people with epilepsy (idiopathic and secondary combined) globally, with an age-standardised prevalence of 658 per 100 000 (569-748). Idiopathic epilepsy had an age-standardised prevalence of 307 per 100 000 (235-389) globally, with 24·2 million (18·5-30·7) prevalent cases, and secondary epilepsy had a global age-standardised prevalence of 350 per 100 000 (322-380). In 2021, 0·7% of the population had active epilepsy (0·3% attributed to idiopathic epilepsy and 0·4% to secondary epilepsy), and the age-standardised global prevalence of epilepsy from idiopathic and secondary epilepsy combined increased from 1990 to 2021 by 10·8% (1·1-21·3), mainly due to corresponding changes in secondary epilepsy. However, age-standardised death and DALY rates of idiopathic epilepsy reduced from 1990 to 2021 (decline of 15·8% [8·8-22·8] and 14·5% [4·2-24·2], respectively). There were three-fold to four-fold geographical differences in the burden of active idiopathic epilepsy, with the bulk of the burden residing in low-income to middle-income countries: 82·1% (81·1-83·4) of incident, 80·4% prevalent (79·7-82·7), 84·7% (83·7-85·1) fatal epilepsy, and 87·9% (86·2-89·2) epilepsy DALYs.
Interpretation: Although the global trends in idiopathic epilepsy deaths and DALY rates have improved in the preceding decades, in 2021 there were almost 52 million people with active epilepsy (24 million from idiopathic epilepsy and 28 million from secondary epilepsy), with the bulk of the burden (>80%) residing in low-income to middle-income countries. Better treatment and prevention of epilepsy are required, along with further research on risk factors of idiopathic epilepsy, good-quality long-term epilepsy surveillance studies, and exploration of the possible effect of stigma and cultural differences in seeking medical attention for epilepsy.
Funding: Bill and Melinda Gates Foundation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests S Afzal reports support for the present manuscript from King Edward Medical University for study material, research articles, valid data sources and authentic real time information for this manuscript. S Afzal also reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from King Edward Medical University and collaborative partners including University of Johns Hopkins, University of California, University of Massachusetts, KEMCAANA, KEMCA_UK; support for attending meetings and/or travel from King Edward Medical University; participation on a Data Safety Monitoring Board or Advisory Board with National Bioethics Committee Pakistan, King Edward Medical University Ethical Review Board, Ethical Review Board Fatima Jinnah Medical University and Sir Ganga Ram Hospital as a Member of the Technical Working Group on Infectious Diseases; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with the Pakistan Association of Medical Editors, the Faculty of Public Health Royal Colleges UK (FFPH) as Fellow, the Society of Prevention, Advocacy And Research, King Edward Medical University. (SPARK), and with the Pakistan Society of Infectious Diseases as a member; other financial or non-financial interest serving as Dean of Public Health and Preventive Medicine King Edward Medical University, as Chief Editor Annals of King Edward Medical University since 2014, as Director of Quality Enhancement Cell King Edward Medical University, as Principal School of Artificial Intelligence at International Level, as Fellow of Faculty of Public Health United Kingdom, as Advisory Board Member and Chair Scientific Session KEMCA-United Kingdom, as Chairperson International Scientific Conference KEMCAANA United States, as Member of the Research and Publications Higher Education Commission HEC Pakistan, as Member of the Research and Journals Committee Pakistan Medical and Dental Council, as Member of the National Bioethics Committee Pakistan, as Member of the Corona Experts Advisory Group (Punjab), as Member Dengue Experts Advisory Group (Punjab), and as Chair of the Punjab Residency Program Research Committee; all outside the submitted work. R Ancuceanu reports consulting fees from Abbvie and Merck Romania; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbvie, Laropharm, Reckitt, and Merck Romania; support for attending meetings and/or travel from Merck Romania; all outside the submitted work. S Bhaskar reports grants or contracts from Japan Society for the Promotion of Science (JSPS), Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) for a Grant-in-Aid for Scientific Research (KAKENHI) (Grant ID: 23KF0126) and from JSPS and the Australian Academy of Science for a JSPS International Fellowship (Grant ID: P23712); leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with Rotary District 9675, Sydney, Australia as District (Chair, Diversity, Equity & Inclusion), Global Health & Migration Hub Community, Global Health Hub Germany, Berlin, Germany (Chair, Founding Member and Manager), PLOS One, BMC Neurology, Frontiers in Neurology, Frontiers in Stroke, Frontiers in Public Health, Journal of Aging Research, Neurology International, Diagnostics, & BMC Medical Research Methodology (Editorial Board Member), College of Reviewers, Canadian Institutes of Health Research (CIHR), Government of Canada (Member), World Headache Society, Bengaluru, India (Director of Research), Cariplo Foundation, Milan, Italy (Expert Adviser/Reviewer), National Cerebral and Cardiovascular Center, Department of Neurology, Suita, Osaka, Japan (Visiting Director), Cardiff University Biobank, Cardiff, UK (Member, Scientific Review Committee), Rotary Reconciliation Action Plan (Chair); all outside the submitted work. S Cortese reports grants or contracts from NIHR and the European Research Agency; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the British Association of Psychopharmacology (BAP), Canadian ADHD Resource Alliance (CADDRA), Medice, and the Association for Child and Adolescent Mental Health (ACAMH); all outside the submitted work. A Hassan reports consulting fees from Novartis, Sanofi Genzyme, Biologix, Merck, Hikma Pharma, Janssen, Inspire Pharma, Future Pharma, and Elixir Pharma; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Allergan, Merck, Biologix, Janssen, Roche, Sanofi Genzyme, Bayer, Hikma Pharma, Al Andalus, Chemipharm, Lundbeck, Inspire Pharma, Future Pharma and Habib Scientific Office, and Everpharma; support for attending meetings and/or travel from Novartis, Allergan, Merck, Biologix, Roche, Sanofi Genzyme, Bayer, Hikma Pharma, Chemipharm, Al Andalus, and Clavita Pharm; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, as vice president of MENA Headache Society, board member of the Headache Chapter of the Egyptian Society of Neurology, board member of Multiple Sclerosis Chapter of the Egyptian Society of Neurology, member of the Committee of Education of the International Headache Society (IHS), membership committee of IHS, and regional committee of IHS; all outside the submitted work. I Ilic reports supports from the present manuscript from the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (project no. 175042, 2011-2023). M Ilic reports support for the present manuscript from the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (project no. 451-03-47/2023-01/200111). K Krishan reports non-financial support from the UGC Centre of Advanced Study, CAS II, awarded to the Department of Anthropology, Panjab University, Chandigarh, India, outside the submitted work. H R Marateb reports grants or contracts paid to their institution from Beatriu de Pinós post-doctoral program from the Office of the Secretary of Universities and Research from the Ministry of Business and Knowledge of the Government of Catalonia program: 2020 BP 00261, outside the submitted work. L Monasta reports support for the present manuscript from the Italian Ministry of Health (Ricerca Corrente 34/2017), payments made to the Institute for Maternal and Child Health IRCCS Burlo Garofolo. S K Panda reports support for the present manuscript via salary from Siksha ‘O’ Anusandhan (Deemed to be University). S K Panda also reports grants or contracts from DST-GOVT. OF ODISHA (Letter No. 3444/ST) outside the submitted work. R Passera reports participation on a Data Safety Monitoring Board or Advisory Board as a member of the Data Safety Monitoring Board of the clinical trial “Consolidation with ADCT-402 (loncastuximab tesirine) after immunochemotherapy: a phase II study in BTKi-treated/ineligible Relapse/Refractory Mantle Cell Lymphoma (MCL) patients” - FIL, Fondazione Italiana Linfomi, Alessandria; leadership or fiduciary roles in board, society, committee or advocacy groups as Member of the EBMT Statistical Committee, European Society for Blood and Marrow Transplantation, Paris (F) (unpaid) and as a past member of 2020-2023 (biostatistician) of the IRB/IEC Comitato Etico AO SS. Antonio e Biagio Alessandria-ASL AL-VC (paid reimbursement of expenses); all outside the submitted work. I Rautalin reports support for the present manuscript from Sigrid Juselius Foundation, Finnish Medical Foundation, Sakari Alhopuro Foundation, Finnish Foundation for Cardiovascular Research, Maud Kuistila Foundation for personal research grants with no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the manuscript's preparation, review, or approval. U Saeed reports support for the present manuscript from the Ontario Graduate Scholarship awarded at the University of Toronto, Canada. Y L Samodra reports leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with Benang Merah Research Center (bmrc.id) as Co-founder, outside the submitted work. V Sharma reports other financial or non-financial support from DFSS (MHA)‘s research project (DFSS28(1)2019/EMR/6) at Institute of Forensic Science & Criminology, Panjab University, Chandigarh, India, outside the submitted work. J A Singh reports consulting fees from ROMTech, Atheneum, Clearview Healthcare Partners, American College of Rheumatology, Yale, Hulio, Horizon Pharmaceuticals, DINORA, ANI/Exeltis, USA Inc., Frictionless Solutions, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care Options, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point Communications, and the National Institutes of Health; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Simply Speaking; support for attending meetings and/or travel from OMERACT as past steering committee member; participation on a Data Safety Monitoring Board or Advisory Board, unpaid, with the FDA Arthritis Advisory Committee; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid, with OMERACT as past steering committee member, the Veterans Affairs Rheumatology Field Advisory Committee as Chair, and the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis as Editor and Director; stock or stock options in Atai Life Sciences, Kintara Therapeutics, Intelligent Biosolutions, Acumen Pharmaceutical, TPT Global Tech, Vaxart pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp., Aebona Pharmaceuticals, and Charlotte's Web Holdings, Inc. and previously-owned stock options in Amarin, Viking and Moderna Pharmaceuticals; all outside the submitted work. R Tabarés-Seisdedos reports grants or contracts from the Valencian Regional Government's Ministry of Education (PROMETEO/CIPROM/2022/58) and the Spanish Ministry of Science, Innovation and Universities (PID2021-129099OB-I00) outside the submitted work. J H V Ticoalu reports leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with Benang Merah Research Center (bmrc.id) as Co-founder, outside the submitted work. S J Tromans reports grants or contracts paid to their institution, University of Leicester, from NHS Digital, via the Department of Health and Social Care as part of the 2023 Adult Psychiatric Morbidity Survey team collecting epidemiological data on community-based adults living in England; leadership or fiduciary roles in other board, society, committee or advocacy groups, paid or unpaid, with the Neurodevelopmental Psychiatry Special Interest Group and Psychiatry of Intellectual Disability Faculty at the Royal College of Psychiatrist as Academic Secretary, with BMC Psychiatry, Advances in Autism, Advances in Mental Health and Intellectual Disability, and Progress in Neurology and Psychiatry as Editorial Board Member, and with Psychiatry of Intellectual Disability Across Cultures (Oxford University Press) as Editor; all outside the submitted work. S Wiebe reports grants or contracts from Alberta Strategy for patient-oriented research and Epilepsy Canada; consulting fees from UCB Pharm, Eisai, Sunovion, and Liva Nova for educational grants paid to their institution; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Torrent Pharma and Biopas; participation on Advisory Boards with Jazz Pharmaceuticals and Paladyn Labs; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with the International League Against Epilepsy as Executive Committee Member; all outside the submitted work. M Zielińska reports other financial or non-financial interests as an AstraZeneca employee outside the submitted work.
Figures
References
-
- Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. - PubMed
-
- WHO . World Health Organization; Geneva: 2019. Epilepsy: a public health imperative.
-
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. - PubMed
-
- Begley C, Wagner RG, Abraham A, et al. The global cost of epilepsy: a systematic review and extrapolation. Epilepsia. 2022;63:892–903. - PubMed
-
- Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54:185–191. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

