Pathogenesis and Management of Intestinal Failure-Associated Liver Disease
- PMID: 40015320
- PMCID: PMC12031023
- DOI: 10.1055/a-2545-7370
Pathogenesis and Management of Intestinal Failure-Associated Liver Disease
Abstract
Long-term parenteral nutrition (PN) has considerably improved the management of intestinal failure (IF) in children and adults, particularly those with short bowel syndrome; however, it carries a significant risk of hepatotoxicity, specifically, intestinal failure-associated liver disease (IFALD), also known as PN-associated liver disease. This review provides an update on the latest understanding of IFALD pathogenesis, emerging therapies, and ongoing challenges in the management of this complication. A number of factors are associated with the development of IFALD. PN lipid emulsions, phytosterol exposure, bacterial dysbiosis, an altered gut-liver axis, and episodes of sepsis disrupt bile acid homeostasis and promote liver inflammation in the active phase of IFALD, favoring the development of PN-associated cholestasis (PNAC) and the more chronic form of steatohepatitis with fibrosis. Based on the identification of pathophysiological pathways, potential therapies are being studied in preclinical and clinical trials, including lipid emulsion modifications; targeted therapies such as Farnesoid X receptor (FXR) and liver receptor homolog 1 (LRH-1) agonists, tumor necrosis factor inhibitors, glucagon-like peptide-2 analogs; microbiome modulation; and supplementation with choline and antioxidants. In conclusion, the pathogenesis of IFALD is complex, and PN dependence and liver injury remain challenging, particularly in patients with IF who cannot advance to enteral nutrition and be weaned off PN.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
None declared.
Figures

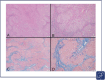
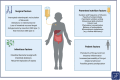

References
-
- Tabone T, Mooney P, Donnellan C. Intestinal failure-associated liver disease: Current challenges in screening, diagnosis, and parenteral nutrition considerations. Nutr Clin Pract. 2024;39(05):1003–1025. - PubMed
-
- Lauriti G, Zani A, Aufieri R et al.Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr. 2014;38(01):70–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

