Essential tremor disrupts rhythmic brain networks during naturalistic movement
- PMID: 40015653
- PMCID: PMC7617547
- DOI: 10.1016/j.nbd.2025.106858
Essential tremor disrupts rhythmic brain networks during naturalistic movement
Abstract
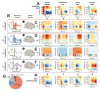
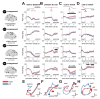
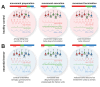
Essential Tremor (ET) is a very common neurological disorder characterised by involuntary rhythmic movements attributable to pathological synchronization within corticothalamic circuits. Previous work has focused on tremor in isolation, overlooking broader disturbances to motor control during naturalistic movements such as reaching. We hypothesised that ET disrupts the sequential engagement of large-scale rhythmic brain networks, leading to both tremor and deficits in motor planning and execution. To test this, we performed whole-head neuroimaging during an upper-limb reaching task using high-density electroencephalography in ET patients and healthy controls, alongside optically pumped magnetoencephalography in a smaller cohort. Key motor regions-including the supplementary motor area, premotor cortex, posterior parietal cortex, and motor cerebellum-were synchronized to tremor rhythms. Patients exhibited a 15 % increase in low beta (14-21 Hz) desynchronization over the supplementary motor area during movement, which strongly correlated with tremor severity (R2 = 0.85). A novel dimensionality reduction technique revealed four distinct networks accounting for 97 % of the variance in motor-related brain-wide oscillations, with ET altering their sequential engagement. Consistent with our hypothesis, the frontoparietal beta network- normally involved in motor planning-exhibited additional desynchronization during movement execution in ET patients. This altered engagement correlated with slower movement velocities, suggesting an adaptation towards feedback-driven motor control. These findings reveal fundamental disruptions in distributed motor control networks in ET and identify novel biomarkers as targets for next-generation brain stimulation therapies.
Keywords: Essential tremor; Motor control; Movement disorders; Oscillations; Reaching.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest T.S. provides paid consultancy for Jazz Pharmaceuticals. The remaining authors have no competing interests to declare. The European Commission support for the production of this publication does not constitute an endorsement of the contents which reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.
Figures




References
-
- Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends in Cognitive Sciences. Elsevier Current Trends. 2001:487–494. - PubMed
-
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–96. - PubMed
-
- Elble RJ, Deuschl G. Current Neurology and Neuroscience Reports. Springer; 2009. An update on essential tremor; pp. 273–277. - PubMed
-
- Raethjen J, Deuschl G. Clinical Neurophysiology. Elsevier; 2012. The oscillating central network of Essential tremor; pp. 61–64. - PubMed
-
- Helmich RC, Toni I, Deuschl G, Bloem BR. The pathophysiology of essential tremor and parkinson’s tremor. Curr Neurol Neurosci Rep. 2013;13:1–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

