Active telomere elongation by a subclass of cancer-associated POT1 mutations
- PMID: 40015989
- PMCID: PMC11960693
- DOI: 10.1101/gad.352492.124
Active telomere elongation by a subclass of cancer-associated POT1 mutations
Abstract
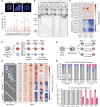
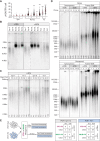
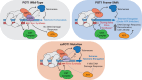
Mutations in the shelterin protein POT1 are associated with diverse cancers and thought to drive carcinogenesis by impairing POT1's suppression of aberrant telomere elongation. To classify clinical variants of uncertain significance (VUSs) and identify cancer-driving loss-of-function mutations, we developed a locally haploid human stem cell system to evaluate >1900 POT1 mutations, including >600 VUSs. Unexpectedly, many validated familial cancer-associated POT1 (caPOT1) mutations are haplosufficient for cellular viability, indicating that some pathogenic alleles do not act through a loss-of-function mechanism. Instead, POT1's DNA damage response suppression and telomere length control are genetically separable. ATR inhibition enables isolation of frameshift mutants, demonstrating that the only essential function of POT1 is to repress ATR. Furthermore, comparison of caPOT1 and frameshift alleles reveals a class of caPOT1 mutations that elongate telomeres more rapidly than full loss-of-function alleles. This telomere length-promoting activity is independent from POT1's role in overhang sequestration and fill-in synthesis.
Keywords: POT1; cancer; deep scanning mutagenesis; pluripotent stem cells; telomerase; telomere.
© 2025 Martin et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
