The MEK inhibitor trametinib is effective in inhibiting the growth of canine oral squamous cell carcinoma
- PMID: 40016294
- PMCID: PMC11868584
- DOI: 10.1038/s41598-025-90574-3
The MEK inhibitor trametinib is effective in inhibiting the growth of canine oral squamous cell carcinoma
Abstract
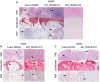
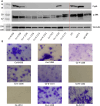
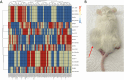
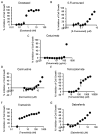
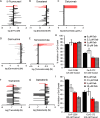
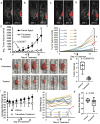
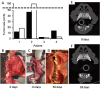
Oral tumors are relatively common in dogs, and canine oral squamous cell carcinoma (COSCC) is the most prevalent oral malignancy of epithelial origin. COSCC is locally aggressive with up to 20% of patients showing regional or distant metastasis at the time of diagnosis. The treatment of choice most typically involves wide surgical excision. Although long-term remission is possible, treatments are associated with considerable morbidity and can negatively impact functionality and quality of life. OSCCs have substantial upregulation of the RAS-RAF-MEK-MAPK signaling axis, and we had previously hypothesized that small-molecule inhibitors that target RAS signaling might effectively inhibit tumor growth and progression. Here, we demonstrate that the MEK inhibitor trametinib, an FDA-approved drug for human cancers, substantially inhibits the growth of six COSCC cell lines established from current patient tumor samples. We further show preliminary clinical evidence that the drug is able to cause ~ 40% and ~ 80% tumor regression in two out of four patients with spontaneously occurring COSCC, a partial response according to commonly used RECIST criteria. Given the limited treatment options available and the number of dogs for which standard of care is not acceptable, these preliminary findings provide new hope that more suitable treatment options may soon enter the veterinary clinic.
Keywords: Canine cancer; Oral squamous cell carcinoma; RAS signaling; Trametinib.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Fulton, A. J., Nemec, A., Murphy, B. G., Kass, P. H. & Verstraete, F. J. M. Risk factors associated with survival in dogs with nontonsillar oral squamous cell carcinoma 31 cases (1990–2010). J. Am. Vet. Med. Assoc.243, 696–702. 10.2460/javma.243.5.696 (2013). - PubMed
-
- Peralta, S., Grenier, J. K., McCleary-Wheeler, A. L. & Duhamel, G. E. Ki67 labelling index of neoplastic epithelial cells differentiates canine acanthomatous ameloblastoma from oral squamous cell carcinoma. J. Comput. Pathol.171, 59–69. 10.1016/j.jcpa.2019.08.001 (2019). - PubMed
-
- Mestrinho, L. A. et al. Comparison of histological and proliferation features of canine oral squamous cell carcinoma based on intraoral location: 36 Cases. J. Vet. Dent.34, 92–99. 10.1177/0898756417713979 (2017). - PubMed
-
- Grimes, J. A. et al. Histologic evaluation of mandibular and medial retropharyngeal lymph nodes during staging of oral malignant melanoma and squamous cell carcinoma in dogs. J. Am. Vet. Med. Assoc.254, 938–943. 10.2460/javma.254.8.938 (2019). - PubMed
-
- Mestrinho, L. A. Current status and future perspectives in canine oral squamous cell carcinoma. Vet. Pathol.55, 200–201. 10.1177/0300985817732114 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

