Daratumumab/lenalidomide/dexamethasone in transplant-ineligible newly diagnosed myeloma: MAIA long-term outcomes
- PMID: 40016302
- PMCID: PMC11976258
- DOI: 10.1038/s41375-024-02505-2
Daratumumab/lenalidomide/dexamethasone in transplant-ineligible newly diagnosed myeloma: MAIA long-term outcomes
Abstract
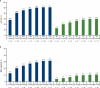
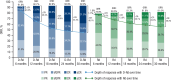
In the MAIA study, daratumumab plus lenalidomide and dexamethasone (D-Rd) improved progression-free survival (PFS) and overall survival (OS) versus lenalidomide and dexamethasone (Rd) alone in transplant-ineligible patients with newly diagnosed multiple myeloma (NDMM). We report updated efficacy and safety from MAIA (median follow-up, 64.5 months), including a subgroup analysis by patient age (<70, ≥70 to <75, ≥75, and ≥80 years). Overall, 737 transplant-ineligible patients with NDMM were randomized 1:1 to D-Rd or Rd. The primary endpoint, PFS, was improved with D-Rd versus Rd (median, 61.9 vs 34.4 months; hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.45-0.67; P < 0.0001). Median OS was not reached in the D-Rd group versus 65.5 months in the Rd group (HR, 0.66; 95% CI, 0.53-0.83; P = 0.0003); estimated 60-month OS rates were 66.6% and 53.6%, respectively. D-Rd achieved higher rates of complete response or better (≥CR; 51.1% vs 30.1%), minimal residual disease (MRD) negativity (32.1% vs 11.1%), and sustained MRD negativity (≥18 months: 16.8% vs 3.3%) versus Rd (all P < 0.0001). D-Rd demonstrated clinically meaningful efficacy benefits across age groups. No new safety concerns were observed. Updated results (median follow-up, >5 years) continue to support frontline use of D-Rd in transplant-ineligible patients with NDMM.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: TF has nothing to disclose. PM served on an advisory board for and received honoraria from Janssen, Celgene, Amgen, AbbVie, Sanofi, and Oncopeptides. KW received research funding from Amgen, Adaptive Biotechnologies, AstraZeneca, Bristol Myers Squibb/Celgene, BeiGene, Janssen, GSK, Sanofi, Stemline, and Takeda; received honoraria from AbbVie, Amgen, Adaptive Biotechnologies, AstraZeneca, Bristol Myers Squibb/Celgene, BeiGene, Janssen, GSK, Karyopharm, Novartis, Oncopeptides, Pfizer, Roche, Sanofi, Stemline, and Takeda; and received travel support from Janssen, GSK, and Sanofi. HG received grants and/or provisions of Investigational Medicinal Product from Amgen, Array BioPharma/Pfizer, Bristol Myers Squibb/Celgene, Chugai, Dietmar Hopp Foundation, Janssen, Johns Hopkins University, Mundipharma, and Sanofi; received research support from Amgen, Bristol Myers Squibb, Celgene, GlycoMimetics, GSK, Heidelberg Pharma, Roche, Karyopharm, Janssen, Incyte, Millennium Pharmaceuticals, Molecular Partners, Merck Sharp & Dohme, MorphoSys AG, Pfizer, Sanofi, Takeda, and Novartis; served on an advisory board for Amgen, Bristol Myers Squibb, Janssen, Sanofi, and Adaptive Biotechnologies; received honoraria from Amgen, Bristol Myers Squibb, Chugai, GSK, Janssen, Novartis, Sanofi, and Pfizer; and received support for attending meetings and/or travel from Amgen, Bristol Myers Squibb, GSK, Janssen, Novartis, Sanofi, and Pfizer. SZU received research funding from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, and Takeda; served in a consulting role for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Edo Pharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, Secura Bio, SkylineDx, Takeda, and TeneoBio; and served on a speakers bureau for Amgen, Bristol Myers Squibb, Janssen, and Sanofi. AC served in a consulting role or on an advisory board for AbbVie, Amgen, Antengene, Celgene/Bristol Myers Squibb, Janssen, Karyopharm, GSK, Genentech, Sanofi, Seattle Genetics, Secura Bio, Shattuck Labs, and Millennium/Takeda; and received research funding from Amgen, Celgene/Bristol Myers Squibb, Janssen, Seattle Genetics, and Millennium/Takeda. TP served as an advisor for Janssen, Celgene, Takeda, Oncopeptides, Genentech, CSL Behring, and AbbVie; and received research support from Janssen, Genmab, Celgene, Takeda, Oncopeptides, Genentech, AbbVie, and Roche. RZO received research funding from Asylia Therapeutics, Biotheryx, Heidelberg Pharma, CARsgen, Celgene/Bristol Myers Squibb, Exelixis, Janssen, Sanofi-Aventis, and Takeda; received honoraria from and served as a member on a board of directors or advisory committee for AbbVie, Biotheryx, Bristol Myers Squibb, Janssen, Karyopharm, Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin, Oncopeptides, Regeneron, Sanofi-Aventis, and Takeda; and holds stock in Asylia Therapeutics. NB served in a consulting or advisory role for and received honoraria from AbbVie, Bristol Myers Squibb/Celgene, FORUS, Genentech, Janssen, Karyopharm, Pfizer, Sanofi, and Takeda; and received research funding from Pfizer and Celgene. SB served as a consultant and on a speakers bureau for Sanofi, Pfizer, and Bristol Myers Squibb. CH received honoraria from Janssen, Bristol Myers Squibb, Amgen, Takeda, and AbbVie. HQ served as a consultant for and received research funding from AbbVie, Bristol Myers Squibb, Karyopharm, Amgen, GSK, and Antengene. MO served as a consultant for Janssen. AP received honoraria from AbbVie, Amgen, Celgene/Bristol Myers Squibb, GSK, Janssen, Sanofi, and Takeda; served as a member on a board or advisory committee for Amgen, Celgene/Bristol Myers Squibb, GSK, Janssen, Pfizer, and Takeda; and received research funding from Takeda. CJ served as a member on a board or advisory committee for Amgen and GSK. CPV received honoraria from Janssen, Bristol Myers Squibb, Sanofi, FORUS, Pfizer, AbbVie, GSK, and Amgen. NR served as a consultant for Bristol Myers Squibb, Janssen, Takeda, Amgen, and GSK; and received research funding from bluebird bio. MT has nothing to disclose. MM served on an advisory board for Celgene/Bristol Myers Squibb, Janssen, Takeda, Amgen, GSK, and Sanofi; received research funding from Janssen and Takeda; and received accommodations from Janssen, Celgene/Bristol Myers Squibb, Takeda, Amgen, and Sanofi. LF received honoraria from Janssen, Sanofi, Amgen, and Takeda; and received funding from Janssen and Amgen. XL received honoraria, research support, and consulting fees from Amgen, Merck, Bristol Myers Squibb, GSK, Janssen, Oncopeptides, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutics, Regeneron, and iTeos. GC received research support from Bristol Myers Squibb/Celgene, Janssen, and Takeda; and served as a consultant for and received honoraria from Amgen, Bristol Myers Squibb/Celgene, Janssen, Millennium/Takeda, Roche, GSK, Oncopeptides, and Sanofi. GW, HP, and FB are employees of Janssen and hold stock in Johnson & Johnson. MK and RC are employees of Janssen. SKK has received research funding from AbbVie, Amgen, Allogene, AstraZeneca, Bristol Myers Squibb, CARsgen, GSK, Janssen, Novartis, Roche-Genentech, Takeda, Regeneron, and Molecular Templates; participated in consulting or an advisory board (with no personal payments) for AbbVie, Amgen, Bristol Myers Squibb, Janssen, Roche-Genentech, Takeda, AstraZeneca, bluebird bio, Epizyme, Secura Bio, Monte Rosa Therapeutics, Trillium, Loxo Oncology, K36, Sanofi, and Arcellx; and participated in consulting or an advisory board (with personal payments) for Oncopeptides, BeiGene, Antengene, and GLH Pharma.
Figures


References
-
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. - PubMed
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124:3474.
-
- Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197:807–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

