Astrocytic cannabinoid receptor 1 promotes resilience by dampening stress-induced blood-brain barrier alterations
- PMID: 40016352
- PMCID: PMC11976283
- DOI: 10.1038/s41593-025-01891-9
Astrocytic cannabinoid receptor 1 promotes resilience by dampening stress-induced blood-brain barrier alterations
Abstract
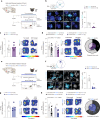
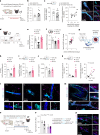
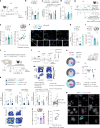
Blood-brain barrier (BBB) alterations contribute to stress vulnerability and the development of depressive behaviors. In contrast, neurovascular adaptations underlying stress resilience remain unclear. Here we report that high expression of astrocytic cannabinoid receptor 1 (CB1) in the nucleus accumbens (NAc) shell, particularly in the end-feet ensheathing blood vessels, is associated with resilience during chronic social stress in adult male mice. Viral-mediated overexpression of Cnr1 in astrocytes of the NAc shell results in baseline anxiolytic effects and dampens stress-induced anxiety- and depression-like behaviors in male mice. It promotes the expression of vascular-related genes and reduces astrocyte inflammatory response and morphological changes following an immune challenge with the cytokine interleukin-6, linked to stress susceptibility and mood disorders. Physical exercise and antidepressant treatment increase the expression of astrocytic Cnr1 in the perivascular region in male mice. In human tissue from male donors with major depressive disorder, we observe loss of CNR1 in the NAc astrocytes. Our findings suggest a role for the astrocytic endocannabinoid system in stress responses via modulation of the BBB.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

