Exploring the Effectiveness of Adjunctive Cenobamate in Focal Epilepsy: A Time-Based Analysis
- PMID: 40016473
- PMCID: PMC11982142
- DOI: 10.1007/s40263-025-01166-8
Exploring the Effectiveness of Adjunctive Cenobamate in Focal Epilepsy: A Time-Based Analysis
Abstract
Background: A growing body of evidence supports the effectiveness of cenobamate (CNB). This study aimed to assess the clinical response to add-on CNB through a time-to-event approach and explore the potential contribution of the concomitant classes of antiseizure medications (ASMs) to improve CNB clinical use.
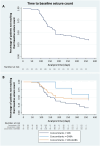
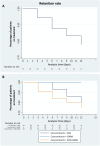
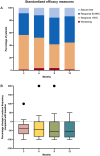
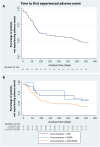
Patients and methods: This study is a subgroup analysis of a larger retrospective, multicenter study on adults with focal-onset seizures participating in the Italian Expanded Access Program at five pre-established centers. The primary endpoint was the time-to-baseline seizure count; secondary endpoints included the rates of seizure response, seizure freedom (defined as no seizures' occurrence since at least the previous follow-up visit), treatment discontinuation, and adverse events (AEs).
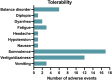
Results: Data on 92 participants were extracted, with a median age of 44 (first quartile (Q1)-third quartile (Q3): 29.25-50.75) years. The number of seizures recorded during the 90-day baseline was reached by 59/92 (64.1%) subjects during the 12-month follow-up. A higher, but not statistically significant probability of reaching the baseline seizures count was shown in the subgroups of subjects taking CNB with sodium channel blockers (SCBs) (hazard ratio [HR] 2.75; 95% confidence interval [CI] 0.79-9.61, p = 0.112) and both SCBs and GABAergics (HR 1.48; 95% CI 0.43-5.09, p = 0.536) compared with subjects taking GABAergics without SCBs. At 12 months, the rates of seizure response, seizure-freedom, and treatment discontinuation were 42.0%, 13.6%, and 23.9%, respectively. A total of 47/92 (51.1%) subjects experienced AEs (mainly somnolence, dizziness, and balance disorders) at a median time of 61 (Q1-Q3: 30-101) days. There was a higher, but not statistically significant risk of AEs occurrence in subjects treated with both SCBs and GABAergics and in those taking SCBs without GABAergics (HR 2.24; 95% CI 0.51-9.82, p = 0.286 and HR 1.40; 95% CI 0.31-6.39, p = 0.661, respectively) compared with those taking GABAergics without SCBs. The main limitations are the retrospective design and the small sample size.
Conclusions: This time-to-event analysis added new insights to the currently available evidence about the real-world effectiveness of add-on CNB. Explorative estimates suggested favorable trends for subjects treated with concomitant GABAergics and without SCBs, who seemed to reach baseline seizure count and experience AEs less frequently and later than subjects treated with other concomitant ASMs. Further studies are needed to identify the best combinations of CNB with other ASMs to maximize seizure control and tolerability.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Author contributions: R.R., E. Russo, and S.L. contributed to the study conception and design. Data collection was performed by V.C., A.D.A., C.D.B., Giancarlo D.G., F.F., E.F., A.M., A.P. E. Rosati, and I.S.; data analysis was performed by R.R. and Gianfranco D.G. The first draft of the manuscript was written by R.R., Gianfranco D.G., E. Russo, and S.L. and all authors commented on previous versions of the manuscript. All authors read and approved of the final manuscript. Funding: Open access funding provided by Università degli studi "Magna Graecia" di Catanzaro within the CRUI-CARE Agreement. Open Access fee was funded by University Magna Grecia of Catanzaro. This research was supported by an unconditional grant from Angelini Pharma. Conflict of interest: R.R. has received consultancy fees from Eisai. A.D. has participated in pharmaceutical industry-sponsored clinical trials for UCB Pharma, has received speaker honoraria from Eisai, UCB, Angelini Pharma, and Neuraxpharm, and has served on advisory boards for Angelini Pharma. C.D.B. has received consulting fees and honoraria from GW Pharmaceuticals, UCB Pharma, Eisai, Angelini Pharma, and Bial outside the submitted work. Giancarlo D.G. has participated on advisory boards and pharmaceutical industry-sponsored symposia for UCB Pharma, Eisai, BIAL, Lusofarmaco, LivaNova, Arvelle, and Angelini Pharma. E. Rosati has received consultancy fees from Angelini, UCB, Jazz Ph, Ecupharma, and Eisai. E. Russo has received speaker fees or funding from and has participated in advisory boards for Arvelle Therapeutics, Angelini Pharma, Eisai, Pfizer, GW Pharmaceuticals, Jazz Pharmaceuticals, UCB, and Lundbeck. S.L. has received speaker’s or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, Medscape, and UCB Pharma and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, Eisai, and GW Pharmaceuticals outside the submitted work. S.L. received research grant support from the Ministry of Health and the Ministry of University and Research outside the submitted work. The remaining authors have no conflicts of interest. S.L. is an Editorial Board member of CNS Drugs. S.L. was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Ethics approval: The overall, anonymized data collection was approved by the Ethics Committee of Calabria Region, Italy (protocol number 416/20) and conducted in accordance with the Declaration of Helsinki. Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request. Consent to participate: All patients provided written informed consent to participate in the study. Consent for publication: Not applicable. Code availability: Not applicable.
Figures
References
-
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official Report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. - PubMed
-
- Asadi-Pooya AA, Brigo F, Lattanzi S, Blumcke I. Adult epilepsy. Lancet. 2023;402:412–24. - PubMed
-
- Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5:e551–67. - PubMed
-
- Perucca E, Perucca P, White HS, Wirrell EC. Drug resistance in epilepsy. Lancet Neurol. 2023;22:723–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

