Melphalan-based conditioning with post-transplant cyclophosphamide for peripheral blood stem cell transplantation: donor effect
- PMID: 40016513
- PMCID: PMC12061766
- DOI: 10.1038/s41409-025-02523-3
Melphalan-based conditioning with post-transplant cyclophosphamide for peripheral blood stem cell transplantation: donor effect
Abstract
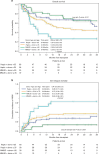
Fludarabine and melphalan (FM) conditioning offers effective disease control with an acceptable toxicity profile. Post-transplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis has improved transplant outcomes. We retrospectively reviewed patients receiving FM-based transplants with PTCy at City of Hope. Of 248 patients included, 89 (35.9%) received hematopoietic cell transplant (HCT) from a matched related/unrelated donor (MRD/MUD), 118 (47.6%) from a haploidentical (HID) donor, and 49 (19.8%) from a mismatched unrelated donor (MMUD). There were no differences in acute and chronic GVHD based on donor type. The 2-year overall survival (OS) for patients receiving HID, MMUD, and MRD/MUD was 58%, 55%, and 70%; disease-free survival (DFS) was 52%, 48%, and 66%; and graft-versus-host/relapse-free survival (GRFS) were 48%, 40%, and 59%, respectively. OS, DFS, and GRFS were similar regardless of donor type on multivariable analysis. However, donor age ≥35 years was associated with lower OS and GRFS and higher 2-year non-relapse mortality (NRM) on multivariable analysis across all patients, regardless of donor type. FM with PTCy appears to produce similar outcomes between MRD/MUD, MMUD, and HID when adjusting for donors <35 years, and donor age seems to be the most important factor when selecting a donor with this regimen.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: PK - Ascentage: Ad board, Daiichi Sankyo: Consulting/Ad Board; BMS: Consulting/Ad Board; Novartis: Consulting/Ad Board; Speakers Bureaul, Travel; Takeda: Ad Board, Speakers Bureau. KS - Autolous: consultancy. IA - Takeda, Adaptive, Syndax, Wugen: consultancy. AA - KiTE and SeaGen: Consultancy. VP - Abbvie: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Amgen: Speakers Bureau; Sobi: Speakers Bureau; Jazz: Speakers Bureau; Alexion: Honoraria; Rigel: Consultancy, Honoraria. MMA - Tscan: Consultancy; NexImmune: Consultancy, Research Funding; CareDx: Consultancy; Incyte: Research Funding; Tr1X: Consultancy. The rest of the authors have no conflicts to disclose.
Figures


References
-
- Ponce DM, Sauter C, Devlin S, Lubin M, Gonzales AM, Kernan NA, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transpl. 2013;19:799–803. - PMC - PubMed
-
- Al Malki MM, Nathwani N, Yang D, Armenian S, Dadwal S, Salman J, et al. Melphalan-Based Reduced-Intensity Conditioning is Associated with Favorable Disease Control and Acceptable Toxicities in Patients Older Than 70 with Hematologic Malignancies Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transpl. 2018;24:1828–35. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

