The Impact of Disease Activity Criteria on Extending Injection Intervals in Real-World Patients with Neovascular Age-Related Macular Degeneration
- PMID: 40016538
- PMCID: PMC11920564
- DOI: 10.1007/s40123-025-01108-z
The Impact of Disease Activity Criteria on Extending Injection Intervals in Real-World Patients with Neovascular Age-Related Macular Degeneration
Abstract
Introduction: In clinical practice, intravitreal anti-vascular endothelial growth factor (anti-VEGF) injection intervals for patients with neovascular age-related macular degeneration (nAMD) are based on disease activity, with active or recurrent disease requiring more frequent injections. Injection interval modification criteria differ from those used in clinical trials, thereby potentially affecting treatment outcomes. This analysis evaluated the potential impact of applying disease activity criteria from recent clinical trials (TENAYA/LUCERNE; HAWK/HARRIER; PULSAR) on the decision to extend injection intervals in real-world patients commenced on faricimab after the loading phase of treatment and at 12 months.
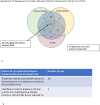
Methods: Data were analysed from 105 treatment-naïve patients with nAMD who received anti-VEGF injections at Moorfields Eye Hospital. Disease activity criteria from TENAYA/LUCERNE, HAWK/HARRIER and PULSAR clinical trials were applied to determine the hypothetical impact on the decision to modify injection intervals at week 12 (fourth injection) and 12-month real-world clinic visits.
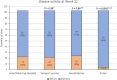
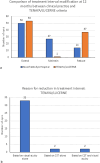
Results: At 12 weeks, 79% of patients had injection intervals extended in clinical practice compared to 80% when applying hypothetical TENAYA/LUCERNE disease activity criteria; 77% using HAWK/HARRIER and 96% using PULSAR. There was agreement between clinical practice and all clinical trials in 60% of eyes, and no agreement in 13%. At 12 months, fewer patients were inactive, with 55% of eyes quiescent in clinical practice, 58% when applying TENAYA/LUCERNE criteria and 67% using HAWK/HARRIER. Application of PULSAR disease activity criteria showed 96% of patients were classed as inactive. 34% of eyes showed agreement in disease activity status between clinical practice and all clinical trials at 12 months, with no agreement in 20%.
Conclusions: Applying disease activity criteria from clinical trials to clinical practice can have a significant impact on hypothetical anti-VEGF injection intervals. Consideration should be paid to which criteria are used in real-world practice to help achieve treatment burden reductions and optimal treatment outcomes seen in clinical trials.
Keywords: Anti-vascular endothelial growth factor (anti-VEGF); Neovascular age-related macular degeneration (nAMD); Optical coherence tomography (OCT); Real-world.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Bhairavi Bhatia: Travel support-Roche. Sing Yue Sim: Consultant—Roche; Travel support—Roche. Evangelia Chalkiadaki: Travel support—Roche. Georgios Koutsocheras: Travel support—Roche. Luke Nicholson: Research grant—Optos; Consultant—Bayer, Abbvie, Roche, Boehringer Ingelheim; Honoraria—Bayer, Roche, Abbvie. Senthil Selvam: Travel support—Bayer, Roche; Honoraria—Bayer, Roche. Sobha Sivaprasad: Financial support—AbbVie, Amgen, Apellis, Bayer, Biogen, Boehringer Ingelheim, Eyebiotech, Eyepoint Phamaceuticals, Janssen Pharmaceuticals, Nova Nordisk, Optos, Ocular Therapeutix, Kriya Therapeutics, OcuTerra, Roche, Stealth Biotherapeutics, Sanofi; Senior Investigator—NIHR; Editor-in-Chief—EYE; Chair—RCOphth Scientific Committee; Trustee—Macular Society. Bishwanath Pal: Consultant—Bayer; Honoraria—Bayer. Josef Huemer: No competing interests. Pearse A Keane: Consultant—Retina Consultants of America, Topcon, Roche, Boehringer-Ingleheim, and Bitfount; Equity owner—Big Picture Medical; Speaker fees-Zeiss, Novartis, Gyroscope, Boehringer-Ingleheim, Apellis, Roche, Abbvie, Topcon, and Hakim Group; Travel support—Bayer, Topcon, Roche; Advisory boards—Boehringer-Ingleheim, RetinAI, Novartis, Apellis. Robin Hamilton: Grants—Bayer, Roche; Consultant—Bayer, Roche; Honoraria—Bayer, Roche; Travel support—Bayer, Roche. Praveen J Patel: Advisory Board: Bayer, Boehringer Ingelheim, Genentech, Inc., Novartis, Roche; Honoraria/Educational Grant: Bayer, Roche. Praveen J Patel is an Editorial Board member of Ophthalmology and Therapy. Praveen J Patel was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Ethical Approval: The study was approved by the Moorfields Eye Hospital Audit Department (Audit number 1319) and followed the tenets of the Declaration of Helsinki of 1964 and its later amendments.
Figures


References
-
- Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34. - PubMed
-
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. - PubMed
-
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40. - PubMed
-
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. - PubMed
-
- Lanzetta P, Korobelnik J-F, Heier JS, et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet. 2024;403:1141–52. - PubMed
LinkOut - more resources
Full Text Sources

