CITRINO: phase 1 dose escalation study of anti-LAG-3 antibody encelimab alone or in combination with anti-PD-1 dostarlimab in patients with advanced/metastatic solid tumours
- PMID: 40016550
- PMCID: PMC11868598
- DOI: 10.1038/s44276-024-00118-x
CITRINO: phase 1 dose escalation study of anti-LAG-3 antibody encelimab alone or in combination with anti-PD-1 dostarlimab in patients with advanced/metastatic solid tumours
Abstract
Background: Dual programmed cell death protein (ligand)-1 (PD-[L]1) and lymphocyte-activation gene-3 (LAG-3) blockade has demonstrated improved anti-tumour response in some advanced solid tumours. CITRINO, a two-part, Phase 1 dose-escalation study, evaluated encelimab (TSR-033; novel anti-LAG-3) monotherapy and in combination in patients with advanced/metastatic solid tumours.
Methods: Part 1 (P1) involved dose escalation (20-720 mg Q2W) of encelimab as monotherapy (P1A/B) and with dostarlimab (500 mg Q3W) in patients with previously treated advanced/metastatic solid tumours (P1C). P2 involved cohort expansion in patients with anti-PD-(L)1-naïve microsatellite stable advanced/metastatic colorectal cancer with recommended phase 2 dose (RP2D) of encelimab with dostarlimab as third/fourth-line therapy (P2A), or with dostarlimab, bevacizumab and mFOLFOX6/FOLFIRI as second-line therapy (P2B). Objectives included RP2D, safety/tolerability, efficacy, pharmacokinetics/pharmacodynamics, and exploratory biomarkers.
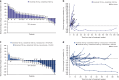
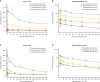
Results: Maximum tolerated encelimab dose was not reached; 720 mg Q2W was used for P2 plus dostarlimab 1000 mg Q6W. One dose-limiting toxicity occurred (Grade 2 myasthenia gravis; P1A). No clinical responses were observed in P1; 1 (3%) and 4 (17%) patients achieved partial response in P2A and 2B, respectively.
Conclusions: Encelimab has a manageable safety profile as a monotherapy and in tested combinations; however, anti-tumour activity was limited.
Clinical trial registration: NCT03250832.
© 2025. The Author(s).
Conflict of interest statement
Ethics approval: This study was conducted in accordance with International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines and the Declaration of Helsinki. This study protocol was reviewed and approved by relevant ethics committees or institutional review boards (IRBs) at each site in accordance with ICH GCP and applicable country-specific requirements. Ethics committees or IRB names (investigator number/centre number) were as follows: Comite de Protection des Peronnes Ile de France XI (362722/242514); University Hospitals Case Medical Centre (434586/247088); Greenville Hospital System (265348/243157); Dana-Farber Cancer IRB (22606/243147); Dana-Farber Cancer IRB (22606/242642); IntegReview IRB (169995/243149); UCLA Office of the Human Research Protection Program (017855/247875); Advarra (012120/243367); Advarra (432930/247089); IntegReview IRB (021368/243145); Advarra (208365/243155); IntegReview IRB (417524/243142); IntegReview IRB (415147/243150); Baylor Scott and White Research Institute Institutional Review Board (202669/247850). All patients provided written informed consent before participation in the study. Competing interests: JRH received consulting fees from Corcept, Astellas, Gilead, Istari, Gritstone, Exelixis, Deciphera, Galvanize, IGM, Taiho, Bristol Myers Squibb, Novartis, Oncolutions, and MBQ Pharma; received payment or honoraria from Research To Practice, Scripps, and MJH; and has stock options in Triumvira, Actym, and MBQ Pharma. JMM received research funding from ASTEX; payment or honoraria from Bristol Myers Squibb; travel support for meeting attendance from GSK; and participated on a Data Safety Monitoring Board or advisory board for Regeneron Pharmaceuticals. DB has nothing to disclose. AP received research funding from GSK. KYC has nothing to disclose. JW received consulting fees from Kanaph Therapeutics and Fusion Pharma; speaker’s bureau and honoraria from AstraZeneca and Eisai. GF received royalties from Wolters Kluwer UK; held an advisory role for AbbVie, Fujifilm, Silicon, Navire, Turning Point, Predicine, Inspirna, Regeneron, Jubilant, BostonGene, Teon, Merck, Sanofi, BridgeBio, and EMD Serono; Speaker’s honorarium from Total Health Conferencing and Rocky Mountain Oncology Society; travel support from Amgen, Bristol Myers Squibb, EMD Serono, Fujifilm, Millennium, Sarah Cannon Research Institute, and Synthorx/Sanofic; received research funding from 3-V Biosciences, Abbisko, Abbvie, ABL Bio, ADC Therapeutics, Accutar, Agenus, Aileron, American Society of Clinical Oncology, Amgen, ARMO/Eli Lilly, Artios, AstraZeneca, Bayer, BeiGene, Bioatla, Bioinvent, Biothera, Bicycle, Black Diamond, Boehringer Ingelheim, Celgene, Celldex, Ciclomed, Curegenix, Curis, Cyteir, Daiichi, DelMar, eFFECTOR, Eli Lilly, EMD Serono, Epizyme, Erasca, Exelixis, Freenome, Fujifilm, Genmab, GSK, Hutchison MediPharma, IGM Biosciences, Ignyta, Immunitas, ImmunoGen/MacroGenics, Incyte, Jacobio, Jazz, Jounce, Jubilant, Kineta, Kolltan, Loxo/Bayer, MedImmune, Merck, Metabomed, Millennium, Mirati, miRNA Therapeutics, Molecular Templates, National Institutes of Health, Navire/BridgeBio, NGM Bio, NiKang, Novartis, OncoMed, Oncorus, Oncothyreon, Poseida, Precision Oncology, Prelude, PureTech, Pyramid, Pyxis, RasCal, Regeneron, Relay, Rgenix, Ribon, Roche, Samumed, Sapience, Seagen, Silicon, Simcha, Sirnaomics, Strategia, Syndax, Synthorx/Sanofi, Taiho, Takeda, Tallac, Tarus, Tarveda, Teneobio, Tesaro, Tocagen, Turning Point, U.T. MD Anderson Cancer Center, Vegenics, Xencor, and Zhuhai Yufan. JMC received research funding from Merus, Roche, Servier, and Bristol Myers Squibb; research support from Merck, AstraZeneca, Esperas Pharma, Bayer, Tesaro, Arcus Biosciences, and Apexigen; honoraria for advisory board participation from Incyte and Blueprint Medicines; and has given educational talks sponsored by Bayer, Merck, AstraZeneca, and Genentech. RK received consulting/advisory fees from AstraZeneca, Exelixis, Ipsen, Eisai, Roche, and Pfizer; and speaker’s bureau fees from Incyte and AstraZeneca. AK has nothing to disclose. OM has nothing to disclose. HY is an employee of GSK; and holds stock/shares with GSK and Treos Bio Limited. CE is an employee of and holds stocks/shares with GSK. AW is an employee of GSK. SG is an employee of GSK. HZ is an employee of GSK. KY is an employee of GSK. SDS is an employee of GSK. IDP is an employee of and holds stocks/shares with GSK. SU received support for the present manuscript from GSK and Tesaro; received grants from AbbVie Inc, Adlai Nortye, ArQule Inc, Artios, AstraZeneca, Atreca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Ciclomed LLC, Erasca, Evelo, Exelexis, G1 Therapeutics Inc, GSK, IGM Biosciences, Immunitas, Incyte, Isofol, Klus Pharma Inc, Macrogenics, Merck Co. Inc, Mersana Therapeutics, OncoMed Pharmaceuticals Inc, Pfizer, Regeneron Pharmaceuticals Inc, Revolution Medicines Inc, Synermore Biologics, Takeda, Tarveda, Tesaro, Tempest Therapeutics, Tvardi, and Vigeo Therapeutics; and participated on a Data Safety Monitoring Board or advisory board for Eisai, AstraZeneca, and IGM biosciences.
Figures
References
-
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. 10.1016/j.immuni.2013.07.012. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
