O-SEMA-FAST: A Prospective, Non-interventional Study Investigating Oral Semaglutide Use in Adults with Type 2 Diabetes Mellitus During Ramadan
- PMID: 40016571
- PMCID: PMC11926314
- DOI: 10.1007/s13300-025-01702-1
O-SEMA-FAST: A Prospective, Non-interventional Study Investigating Oral Semaglutide Use in Adults with Type 2 Diabetes Mellitus During Ramadan
Abstract
Introduction: Oral semaglutide, a glucagon-like peptide 1 receptor agonist, requires administration on an empty stomach with up to 120 mL of water, followed by no intake of food, beverages, or other oral medications for at least 30 min to ensure optimal absorption. These instructions can be challenging to adhere to during Ramadan when patients fast for extended periods. The O-SEMA-FAST study assessed the impact of fasting on adherence to oral semaglutide dosing instructions and its subsequent effects on glycaemic control and body weight.
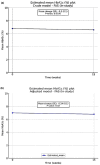
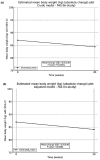
Methods: O-SEMA-FAST was a non-interventional, prospective study conducted in 2023 in people with type 2 diabetes mellitus (T2DM) who fasted during Ramadan and were on oral semaglutide treatment in the United Arab Emirates, Saudi Arabia, and Kuwait. Patients were followed for 20 weeks. Glycated haemoglobin (HbA1c) and body weight were measured at baseline and at the end of the study (EOS); changes were analysed by mixed models for repeated measures.
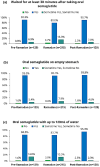
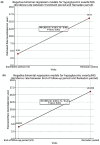
Results: Among the 257 patients included in the final analysis, there was a significant reduction in HbA1c (- 0.2%-points, p = 0.01) and a notable decrease in body weight (- 2.6 kg, p < 0.0001) from baseline to EOS. Of the 215 patients who recorded administration details in their diaries, 68.4% (n = 147) adhered to dosing instructions for ≥ 80% of diary days. Baseline mean HbA1c was 6.7% in adherent patients and 7.0% in non-adherent patients. At EOS, the change in HbA1c was - 0.3%-points (95% confidence interval, CI - 0.4, - 0.2; p < 0.0001) for adherent patients and - 0.1%-points (95% CI - 0.4, 0.1; p = 0.3) for non-adherent patients. The change in body weight was - 3.2 kg (95% CI - 4.0, - 2.4; p < 0.0001) for adherent patients and - 1.6 kg (95% CI - 2.5, - 0.8; p = 0.0001) for non-adherent patients. An increase in self-reported hypoglycaemic events (HEs) was observed, but no severe events were reported. Gastrointestinal disorders were the most common adverse effects. Among patients with available data on self-reported HEs (n = 216), 67 (31.0%) experienced HEs. The mean age, HbA1c levels and T2DM duration of patients with vs without HEs were 51.0 vs 53.3 years, 6.99 vs 6.66% and 9.2 vs 7.9 years. A greater proportion of patients experiencing HEs were treated with oral antidiabetic drugs like biguanides (90.6% vs 86.7%), sodium glucose cotransporter 2 inhibitors (85.9% vs 80.7%), sulfonylureas (32.8% vs 25.9%) and dipeptidyl peptidase 4 inhibitors (20.3% vs 11.1%).
Conclusion: The O-SEMA-FAST study demonstrated that most participants adhered to oral semaglutide instructions and experienced significant reductions in HbA1c and body weight. Overall, baseline characteristics were similar regardless of HEs; however, patients reporting HEs were younger, had higher HbA1c levels, longer T2DM duration and were under polypharmacy. Oral semaglutide is a suitable choice for individuals who fast during Ramadan, effectively controlling glycaemic levels and managing body weight while maintaining a favourable safety profile.
Trial registration: NCT05716724.
Keywords: Adherence; Body weight management; Fasting; Glucagon-like peptide 1 receptor agonist; Glycaemic control; Middle East; Oral semaglutide; Ramadan; Real-world study; Type 2 diabetes mellitus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Mohamed Hassanein has received honorarium from Novo Nordisk, Eli Lilly, Sanofi, Novartis and MSD; has received Lecture/Other Fees from Novo Nordisk, Eli Lilly, Sanofi, Novartis and MSD and he is a principal investigator. Fatheya Al Awadi has nothing to disclose. Ibrahim AlKadim has received honorarium from Novo Nordisk and Eli Lilly; has received Lecture/Other Fees from Novo Nordisk, AstraZeneca, Sanofi, Eli Lilly, Boehringer Ingelheim, SPIMACO and he has shares in Novo Nordisk and Eli Lilly. Hazem Aly is an employee of Novo Nordisk. Dalila Bajawi has received honorarium from Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Sanofi, Eli Lilly, Novartis, Bayer, Merck, Organon, Hikma, Tabuk and participated in trials sponsored by Novo Nordisk and Sanofi. Tarhan Cinar is an employee of Novo Nordisk. Dinesh Dhanwal has received honorarium from Novo Nordisk, Lilly, MSD, Boehringer Ingelheim, Hikma, AstraZeneca and participated in trials sponsored by Novo Nordisk and Lilly, Abdul Jabbar has received honorarium from Novo Nordisk, Lilly, Merck, Boehringer Ingelheim, Abbott, Novartis and participated in trials sponsored by Novo Nordisk, Said Khader has received honorarium from Novo Nordisk, BI, Sanofi, Lilly, AstraZeneca and participated in trials sponsored by Novo Nordisk and Sanofi. Khaled Khudadah has received honorarium from Novo Nordisk. Talal Muzaffar has nothing to disclose. Mary Ngome is an employee of Novo Nordisk. Jalal Nafach has received honorarium Novo Nordisk, Sanofi, MSD, Boehringer Ingelheim, Pfizer, AstraZeneca, P&G and participated in trials sponsored by Novo Nordisk, Sanofi, AstraZeneca, received non-financial support from Novo Nordisk, Amna Shaghouli has received honorarium from Novo Nordisk, Eli Lilly and Boehringer Ingelheim. Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki. Approval was obtained from local institutional review boards, ethics committees and regulatory authorities (if applicable). All patients provided informed written consent to participate in the study. The institutional review boards included Jeddah Central Ministry of Health Institutional Review Board, Al Mouwasat Institutional Review Board, Sulaiman Al Habib central Institutional Review Board, King Fahad Medical City (KFMC), Almoosa hopital Institutional Review Board, Gulf Medical University Institutional Review Board, National Medical Commission (NMC) local Institutional Review Board. Regulatory bodies included Ministry of Health (MOH), Saudi Food and Drug Authority (SFDA), Dubai Health Authority (DHA), Ministry of Health and Prevention of the United Arab Emirates (MOHAP). The study is registered with ClinicalTrials.gov (NCT05716724).
Figures

References
-
- International Diabetes Federation. IDF Diabetes Atlas. https://diabetesatlas.org/. Accessed 2024 Jan 26.
-
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. - PubMed
-
- Hassanein M, Al Sifri S, Shaikh S, et al. A real-world study in patients with type 2 diabetes mellitus treated with gliclazide modified-release during fasting: DIA-RAMADAN. Diabetes Res Clin Pract. 2020;163: 108154. - PubMed
-
- CHMP. Rybelsus® (semaglutide): EU Summary of Product Characteristics (SPC). https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus. Accessed 17 Jan 2025.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

