From low remission to hope: the efficacy of targeted therapies in NUP98-R positive pediatric acute myeloid leukemia
- PMID: 40016600
- PMCID: PMC11958374
- DOI: 10.1007/s12519-025-00875-w
From low remission to hope: the efficacy of targeted therapies in NUP98-R positive pediatric acute myeloid leukemia
Abstract
Background: Treating pediatric acute myeloid leukemia (AML) with NUP98 rearrangement (NUP98-R) is challenging. Standard chemotherapy results in low remission rates. This study aimed to evaluate different induction regimens and explore alternative therapies to improve outcomes.
Methods: This retrospective study included 111 pediatric patients with AML treated at our institution from March 2012 to March 2023. Patients were classified into two groups: NUP98-R-positive (n = 10) and NUP98-R-negative (n = 101). We compared their clinical characteristics, treatment responses, and prognoses. Additionally, we presented three cases of NUP98-R-positive patients to elaborate on the role of targeted therapies during induction in treatment outcomes and prognosis.
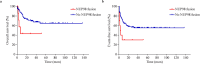
Results: Patients with NUP98-R fusion genes had a complete remission (CR) rate of 20% after the first induction, which was significantly lower than the 64.3% reported in those without NUP98-R fusion genes (P < 0.05). The 3-year event-free survival (EFS) rate was also lower, with only 30% for NUP98-R patients and 55.3% for non-NUP98-R patients (P < 0.05). The prognosis of NUP98-R patients improved with targeted therapies during induction. For example, Patient 1 achieved CR with FLT3 and BCL-2 inhibitors plus conventional chemotherapy. Patient 2, who was treated with a CDK6 inhibitor, a BCL-2 inhibitor, azacitidine, and an FLT3 inhibitor, also achieved CR and underwent successful stem cell transplantation. Conversely, Patient 3, who received only standard chemotherapy, did not achieve remission and died from a severe infection.
Conclusions: This study demonstrated that using targeted drugs for the induction in NUP98-R pediatric AML improved treatment outcomes. BCL-2, FLT3, and CDK6 inhibitors available at our institution are promising options for this phase of treatment.
Keywords: Acute myeloid leukemia; Pediatric; Targeted therapies; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Author Run-Ming Jin is the editorial board member of World Journal of Pediatrics. The paper was handled by the other Editor and has undergone rigrous peer review process. Author Run-Ming Jin was not involved in the journal's review of, or decisions related to, this manuscript. Ethical approval: This study was approved by the Medical Ethics Committee of the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (No. 2024–0753). Written consent for the publication of details of three cases was obtained from the patients’ parents.
Figures

References
-
- Struski S, Lagarde S, Bories P, Puiseux C, Prade N, Cuccuini W, et al. NUP98 is rearranged in 3.8% of pediatric AML forming a clinical and molecular homogenous group with a poor prognosis. Leukemia. 2017;31:565–72. - PubMed
-
- Bertrums EJM, Smith JL, Ries RE, Alonzo TA, Ostronoff F, Kaspers GJL, et al. The molecular characteristics and clinical relevance of NUP98-Other translocations in pediatric acute myeloid leukemia. Blood. 2020;136(Supplement 1):36–7.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

