Application of Box-Behnken design to optimize the phosphorus removal from industrial wastewaters using magnetic nanoparticles
- PMID: 40016607
- PMCID: PMC11928393
- DOI: 10.1007/s11356-025-36152-6
Application of Box-Behnken design to optimize the phosphorus removal from industrial wastewaters using magnetic nanoparticles
Abstract
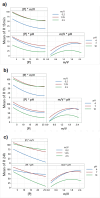
Phosphorus is essential for all living organisms and limits aquatic plant growth. Pulp mill effluents, particularly from Eucalyptus bleached kraft pulp mills, contain phosphorus concentrations that vary with operational conditions. This variability poses challenges for effective treatment and phosphorus removal. However, uncontrolled release of phosphorus-rich wastewaters causes eutrophication. This study focuses on optimizing phosphorus removal from such effluents using cobalt ferrite nanoparticles, with an emphasis on process optimization to address this variability. Minimizing phosphorus concentrations is crucial in wastewater engineering and surface water management. By employing design of experiments and response surface methodology, we aim to fine-tune the phosphorous removal process and pinpoint the key factors with the most significant impact. Optimal conditions for achieving over 90% removal from an effluent with 5 mg P/L were identified as a sorbent dose greater than 1.3 g/L and a pH range between 5 and 7, all within a contact time of only 15 min. For a contact time of 1 and 24 h, the conditions adjust to a sorbent dose greater than 0.97 and 0.83 g/L, respectively, with the pH range remaining the same. Our results highlight the effectiveness of cobalt ferrite nanoparticles as sorbents in the removal of phosphorus for water treatment purposes. This approach presents a sustainable and proficient strategy for phosphorus recovery from pulp mill effluents, thereby lessening environmental repercussions and offering a valuable resource for future use. This contributes to the maintenance of water quality and ecosystem preservation.
Keywords: Adsorption; Box-Behnken design; Cobalt ferrite nanoparticles; Design of experiments; Eutrophication; Phosphate; Response surface methodology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Consent to participate: Not applicable. Consent to publish: All authors have read and approved the manuscript. Competing interests: The authors declare no competing interests.
Figures


References
-
- Ahmed S, Ashiq MN, Li D, Tang P, Leroux F, Feng Y (2019) Recent progress on adsorption materials for phosphate removal. Recent Pat Nanotechnol 13:3–16 - PubMed
-
- Ayoub GM, Kalinian H, Zayyat R (2019) Efficient phosphate removal from contaminated water using functional raw dolomite powder. SN Appl Sci 1:802
-
- Bańkowska-Sobczak A (2021) Calcite as a candidate for non-invasive phosphorus removal from lakes. Ecohydrol Hydrobiol 21:683–699
-
- Chen Y, Xu R, Li Y, Liu Y, Wu Y, Chen Y, Zhang J, Chen S, Yin H, Zeng Z, Wang S, Peng Z (2020) La(OH)3-modified magnetic CoFe2O4 nanocomposites: a novel adsorbent with highly efficient activity and reusability for phosphate removal. Colloids Surf A Physicochem Eng Asp 599:124870–124880
MeSH terms
Substances
Grants and funding
- 2021.06684.BD/Fundação para a Ciência e a Tecnologia
- UIDB/50011/2020 (DOI 10.54499/UIDB/50011/2020)/Fundação para a Ciência e a Tecnologia
- UIDP/50011/2020 (DOI 10.54499/UIDP/50011/2020)/Fundação para a Ciência e a Tecnologia
- LA/P/0006/2020 (DOI 10.54499/LA/P/0006/2020)/Fundação para a Ciência e a Tecnologia
- UIDB/50006/2020 (DOI 10.54499/UIDB/50006/2020)/Fundação para a Ciência e a Tecnologia
LinkOut - more resources
Full Text Sources

