MTHFD2 promotes osteoclastogenesis and bone loss in rheumatoid arthritis by enhancing CKMT1-mediated oxidative phosphorylation
- PMID: 40016725
- PMCID: PMC11866863
- DOI: 10.1186/s12916-025-03945-y
MTHFD2 promotes osteoclastogenesis and bone loss in rheumatoid arthritis by enhancing CKMT1-mediated oxidative phosphorylation
Abstract
Background: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by disrupted bone homeostasis. This study investigated the effect and underlying mechanisms of one-carbon metabolism enzyme methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) on osteoclast differentiation and bone loss in RA.
Methods: The expression of MTHFD2 was examined in CD14 + monocytes and murine bone marrow-derived macrophages (BMMs). RNA-sequencing was performed to evaluate the regulatory mechanisms of MTHFD2 on osteoclastogenesis. Extracellular flux assay, JC-1 staining, and transmission electron microscopy were used to detect mitochondrial function and energy metabolism changes during osteoclast formation. Collagen-induced arthritis (CIA) mice were used to evaluate the therapeutic effect of MTHFD2 knockdown on bone loss. Bone volume and osteoclast counts were quantified by μCT and histomorphometry.
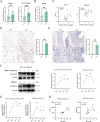
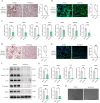
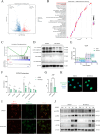
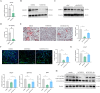
Results: Elevated MTHFD2 was observed in RA patients and CIA mice with a positive correlation to bone resorption parameters. During osteoclast formation, MTHFD2 was significantly upregulated in both human CD14 + monocytes and murine BMMs. The application of MTHFD2 inhibitor and MTHFD2 knockdown suppressed osteoclastogenesis, while MTHFD2 overexpression promoted osteoclast differentiation in vitro. RNA-sequencing revealed that MTHFD2 inhibition blocked oxidative phosphorylation (OXPHOS) in osteoclasts, leading to decreased adenosine triphosphate (ATP) production and mitochondrial membrane potential without affecting mitochondrial biogenesis. Mechanistically, inhibition of MTHFD2 downregulated the expression of mitochondrial creatine kinase 1 (CKMT1), which in turn affected phosphocreatine energy shuttle and OXPHOS during osteoclastogenesis. Further, a therapeutic strategy to knock down MTHFD2 in knee joint in vivo ameliorated bone loss in CIA mice.
Conclusions: Our findings demonstrate that MTHFD2 is upregulated in RA with relation to joint destruction. MTHFD2 promotes osteoclast differentiation and arthritic bone erosion by enhancing mitochondrial energy metabolism through CKMT1. Thus, targeting MTHFD2 may provide a potential new therapeutic strategy for tackling osteoclastogenesis and bone loss in RA.
Keywords: Methylenetetrahydrofolate dehydrogenase 2; Osteoclast differentiation; Oxidative phosphorylation; Rheumatoid arthritis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments were approved by the Medical Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China (2023KY-438). Experiments were conducted in accordance with relevant institutional guidelines and regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Smith MH, Berman JR. What Is Rheumatoid Arthritis? JAMA. 2022;12:1194. - PubMed
-
- Gough AK, et al. Generalised bone loss in patients with early rheumatoid arthritis. Lancet. 1994;8914:23–7. - PubMed
-
- Welsing PM, et al. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;9:2009–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

