Chronic Inflammation in Asthma: Looking Beyond the Th2 Cell
- PMID: 40016948
- PMCID: PMC11868696
- DOI: 10.1111/imr.70010
Chronic Inflammation in Asthma: Looking Beyond the Th2 Cell
Abstract
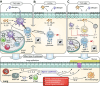
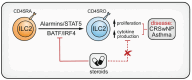
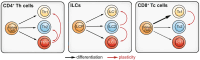
Asthma is a common chronic inflammatory disease of the airways. A substantial number of patients present with severe and therapy-resistant asthma, for which the underlying biological mechanisms remain poorly understood. In most asthma patients, airway inflammation is characterized by chronic activation of type 2 immunity. CD4+ T helper 2 (Th2) cells are the canonical producers of the cytokines that fuel type 2 inflammation: interleukin (IL)-4, IL-5, IL-9, and IL-13. However, more recent findings have shown that other lymphocyte subsets, in particular group 2 innate lymphoid cells (ILC2s) and type 2 CD8+ cytotoxic T (Tc2) cells, can also produce large amounts of type 2 cytokines. Importantly, a substantial number of severe therapy-resistant asthma patients present with chronic type 2 inflammation, despite the high sensitivity of Th2 cells for suppression by corticosteroids-the mainstay drugs for asthma. Emerging evidence indicates that ILC2s and Tc2 cells are more abundant in severe asthma patients and can adopt corticosteroid-resistance states. Moreover, many severe asthma patients do not present with overt type 2 airway inflammation, implicating non-type 2 immunity as a driver of disease. In this review, we will discuss asthma pathophysiology and focus on the roles played by ILC2s, Tc2 cells, and non-type 2 lymphocytes, placing special emphasis on severe disease forms.
Keywords: ILC2; Tc2 cell; Th2 cell; asthma; cytokines; type 2 inflammation.
© 2025 The Author(s). Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Beasley R., Harper J., Bird G., Maijers I., Weatherall M., and Pavord I. D., “Inhaled Corticosteroid Therapy in Adult Asthma. Time for a New Therapeutic Dose Terminology,” American Journal of Respiratory and Critical Care Medicine 199, no. 12 (2019): 1471–1477, 10.1164/rccm.201810-1868CI. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

