High Antigenicity for Treg Cells Confers Resistance to PD-1 Blockade Therapy via High PD-1 Expression in Treg Cells
- PMID: 40017103
- PMCID: PMC12044662
- DOI: 10.1111/cas.70029
High Antigenicity for Treg Cells Confers Resistance to PD-1 Blockade Therapy via High PD-1 Expression in Treg Cells
Abstract
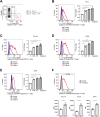
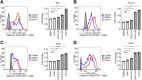
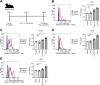
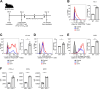
Regulatory T (Treg) cells have an immunosuppressive function, and programmed death-1 (PD-1)-expressing Treg cells reportedly induce resistance to PD-1 blockade therapies through their reactivation. However, the effects of antigenicity on PD-1 expression in Treg cells and the resistance to PD-1 blockade therapy remain unclear. Here, we show that Treg cells gain high PD-1 expression through an antigen with high antigenicity. Additionally, tumors with high antigenicity for Treg cells were resistant to PD-1 blockade in vivo due to PD-1+ Treg-cell infiltration. Because such PD-1+ Treg cells have high cytotoxic T lymphocyte antigen (CTLA)-4 expression, resistance could be overcome by combination with an anti-CTLA-4 monoclonal antibody (mAb). Patients who responded to combination therapy with anti-PD-1 and anti-CTLA-4 mAbs sequentially after primary resistance to PD-1 blockade monotherapy showed high Treg cell infiltration. We propose that the high antigenicity of Treg cells confers resistance to PD-1 blockade therapy via high PD-1 expression in Treg cells, which can be overcome by combination therapy with an anti-CTLA-4 mAb.
Keywords: CTLA‐4; PD‐1; antigenicity; cancer immunotherapy; regulatory T cell.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
K. Ninomiya received honoraria from Ono Pharmaceutical, Bristol‐Myers Squibb, Chugai Pharmaceutical, AstraZeneca, Daiichi‐Sankyo, MSD, Kyowa Kirin, Lilly, Takeda Pharmaceutical, Nippon Kayaku, Pfizer, Janssen Pharmaceutical, Boehringer Ingelheim, Taiho Pharmaceutical, Amgen, Elekta, and CareNet outside this study. T. Inozume received honoraria from Ono Pharmaceutical, Bristol‐Myers Squibb, and MSD; and research grants from Maruho, Taiho, Daiichi‐Sankyo, Torii, and Sun Pharma outside this study. K. Ohashi received honoraria from Boehringer Ingelheim, Chugai Pharmaceutical, Pfizer, Eli Lilly, Nippon Kayaku, KYOWA KIRIN, AstraZeneca, MSD, Novartis, Insmed, and Elekta; and research grants from Boehringer Ingelheim and Chugai Pharmaceutical outside of this study. Y. Togashi received honoraria from Ono Pharmaceutical, Bristol‐Myers Squibb, Chugai Pharmaceutical, AstraZeneca, Eisai, and MSD; and research grants from Ono Pharmaceutical, Bristol‐Myers Squibb, Daiichi‐Sankyo, Janssen Pharmaceutical, AstraZeneca, KOTAI Biotechnologies Inc., and KORTUC outside this study, is the Associate Editor of Cancer Science. All the other authors declare that they have no competing financial interests.
Figures



References
-
- Doki Y., Ajani J. A., Kato K., et al., “Nivolumab Combination Therapy in Advanced Esophageal Squamous‐Cell Carcinoma,” New England Journal of Medicine 386 (2022): 449–462. - PubMed
-
- Hellmann M. D., Paz‐Ares L., Bernabe Caro R., et al., “Nivolumab Plus Ipilimumab in Advanced Non‐Small‐Cell Lung Cancer,” New England Journal of Medicine 381 (2019): 2020–2031. - PubMed
-
- Baas P., Scherpereel A., Nowak A. K., et al., “First‐Line Nivolumab Plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): A Multicentre, Randomised, Open‐Label, Phase 3 Trial,” Lancet 397 (2021): 375–386. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

