Serious Neurologic Adverse Events in Tofersen Clinical Trials for Amyotrophic Lateral Sclerosis
- PMID: 40017137
- PMCID: PMC12060635
- DOI: 10.1002/mus.28372
Serious Neurologic Adverse Events in Tofersen Clinical Trials for Amyotrophic Lateral Sclerosis
Abstract
Introduction/aims: Tofersen is approved for the treatment of amyotrophic lateral sclerosis (ALS) due to superoxide dismutase 1 mutations (SOD1-ALS). Here we report serious neurologic adverse events (AEs) that occurred in the tofersen clinical trials in people with SOD1-ALS.
Methods: Serious neurologic AEs of myelitis, radiculitis, aseptic meningitis, and papilledema reported in the tofersen clinical trials are described. Serious AEs were defined according to International Conference for Harmonization guidelines, and neurologic AEs in clinical trials were diagnosed by investigators based on symptoms, clinical examination findings, and diagnostic workup.
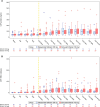
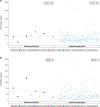
Results: Ten participants (approximately 7% of tofersen 100-mg-treated trial participants) experienced a total of 12 serious neurologic AEs-4 of myelitis, 2 of radiculitis, 2 of aseptic meningitis, and 4 of intracranial hypertension (ICH) and/or papilledema. All events but one resolved either spontaneously, with dosing interruption/modification, or with concomitant therapies. One event was ongoing but improved as of December 2022. While 3 events led to tofersen treatment discontinuation, all other participants were able to remain on treatment. No event was life-threatening or fatal.
Discussion: Some antisense oligonucleotides (ASOs) have been described as having pro-inflammatory properties. Aseptic meningitis has been reported with nusinersen; however, myelitis, radiculitis, increased intracranial pressure, and papilledema have not been reported with ASO treatment. These neurologic AEs should be considered when assessing the overall benefit/risk of tofersen treatment for SOD1-ALS. Safety data from the open-label extension and expanded access program will continue to characterize these events and further inform the safety profile of tofersen in SOD1-ALS.
Keywords: SOD1‐ALS; VALOR; myelitis; serious adverse events; tofersen.
© 2025 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
Alexandra Lovett was an employee of Biogen at the time the study was conducted; Sowmya Chary is an employee of Biogen and may hold stock in the company; Suma Babu has received research funding from NIH, OrphAI Therapeutics, Biogen, Ionis, Novartis, Denali; institutional consulting fees from OrphAI therapeutics, Biogen, Uniqure and MarvelBiome; and platform trial coordination and center activities from HEALEY ALS, NIH‐NINDS. Gaëlle Bruneteau served as a site investigator for several clinical trials sponsored by Biogen; Jonathan D. Glass' institution was contracted by Biogen as a trial site and was paid for those services; received grants from MDA and NIH ALSA; and received commercial sponsorships for clinical trials from Amylyx, Biogen, Cytokinetics, and Neuralstem; Merete Karlsborg reports no disclosures relevant to the manuscript; Shafeeq Ladha serves as a consultant to, and is on the advisory board for, Biogen; and served as a site investigator for clinical trials sponsored by Biogen; Keith Mayl served as an investigator for several clinical trials sponsored by Biogen; Christopher McDermott received grants from MND Association and NIHR; and served as a consultant for Amylyx, Ferrer, Orion Pharma, and Orphazyme; Robert C. Bucelli serves as a consultant to, and is on the advisory board for, Biogen; and served as a site investigator for clinical trials sponsored by Biogen; Adriano Chiò received grants/contracts from Biogen; received consulting fees from Biogen, Cytokinetics, Denali Pharma, Lilly, and Mitsubishi Tanabe; and received payment/honoraria for Amylyx, Biogen, Cytokinetics, and DSMB for ABScience and AL‐S Pharma AG; Toby A. Ferguson was an employee of Biogen at the time the study was conducted; Thos Cochrane was an employee of Biogen at the time the study was conducted; Stephanie Fradette is an employee of Biogen and may hold stock in the company; Karen Smirnakis is an employee of Biogen and may hold stock in the company; Jennifer Inra is an employee of Biogen and may hold stock in the company; Sohail Malek is an employee of Biogen and may hold stock in the company; Laura Fanning was an employee of Biogen at the time the study was conducted.
Figures

References
-
- Bunton‐Stasyshyn R. K., Saccon R. A., Fratta P., and Fisher E. M., “SOD1 Function and Its Implications for Amyotrophic Lateral Sclerosis Pathology: New and Renascent Themes,” Neuroscientist 21, no. 5 (2015): 519–529. - PubMed
-
- Sau D., De Biasi S., Vitellaro‐Zuccarello L., et al., “Mutation of SOD1 in ALS: A Gain of a Loss of Function,” Human Molecular Genetics 16, no. 13 (2007): 1604–1618. - PubMed
-
- Kurreck J., “Antisense Technologies. Improvement Through Novel Chemical Modifications,” European Journal of Biochemistry 270, no. 8 (2003): 1628–1644. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

