Acid Ceramidase Deficiency: New Insights on SMA-PME Natural History, Biomarkers, and In Cell Enzyme Activity Assay
- PMID: 40017560
- PMCID: PMC11867579
- DOI: 10.1212/NXG.0000000000200243
Acid Ceramidase Deficiency: New Insights on SMA-PME Natural History, Biomarkers, and In Cell Enzyme Activity Assay
Abstract
Background and objectives: Spinal muscular atrophy with progressive myoclonic epilepsy (SMA-PME) due to acid ceramidase deficiency is a rare disorder, allelic with Farber disease, resulting from recessive ASAH1 variants. Patients present in early childhood with muscle weakness due to anterior horn degeneration and/or progressive drug-resistant myoclonic epilepsy. Death usually results from respiratory complications or status epilepticus during adolescence.
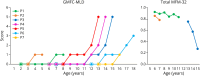
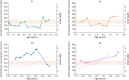
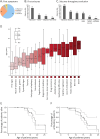
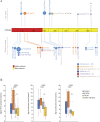
Methods: We identified 9 patients with SMA-PME from 5 different families followed in neurology, rehabilitation, and genetics departments of university hospitals in France and the United States. During disease progression, motor functional scores were assessed for seven of them and C26-ceramide quantification on dried blood spots (DBSs) was performed for 4 of them. An in cell assay, measuring the degradation rate of ceramides in living skin fibroblasts, was also performed in 2 patients. Finally, a literature review was conducted.
Results: Twelve years after the molecular characterization of SMA-PME, here we present the detailed history of 9 patients from 5 different families with 4 new ASAH1 variants. The prospective follow-up for 4 of them allows us to evaluate the relevance of functional scales and of C26-ceramide assay on DBS, as a biomarker. In addition, an in cell assay could provide a more reliable level of the residual ceramidase activity. Based on a comprehensive literature review, we provide a detailed description of the natural history of the 44 patients with SMA-PME diagnosed to date and show a genotype-phenotype correlation for the 2 main variants and the disease onset.
Discussion: This study presents the detailed natural history of SMA-PME. Given the rarity of this disease and the current lack of a reliable biomarker for patient follow-up, this work may serve as a retrospective control group for future therapeutic trials.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
Quantification of C26-ceramide on DBSs was carried out by Camille Moreau, Adrien Paquot, and Terence Beghyn from the biotechnology company APTEEUS, and funded by the patient organization ASAP for Children. The other authors have no conflicts of interest to declare. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
