Trifluridine-tipiracil in previously treated patients with oestrogen receptor-positive, HER2-negative metastatic breast cancer (BOOG 2019-01 TIBET trial): a single-arm, multicentre, phase 2 trial
- PMID: 40017682
- PMCID: PMC11867194
- DOI: 10.1016/j.eclinm.2024.103065
Trifluridine-tipiracil in previously treated patients with oestrogen receptor-positive, HER2-negative metastatic breast cancer (BOOG 2019-01 TIBET trial): a single-arm, multicentre, phase 2 trial
Abstract
Background: Effective later-line chemotherapy treatment options are scarce for patients with metastatic breast cancer (MBC). Trifluridine-tipiracil has shown survival benefit in heavily pre-treated patients with metastatic colorectal and in gastric cancer refractory to a fluoropyrimidine. This study aimed to investigate the efficacy of trifluridine-tipiracil in a Western population of previously treated patients with oestrogen receptor (ER+), HER2- MBC to facilitate further optimization of this treatment strategy.
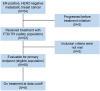
Methods: Adult patients at least 18 years old diagnosed with hormone receptor positive, HER2- receptor negative MBC with a performance status of 0 or 1 who have been treated with capecitabine in the metastatic setting and up to two other lines of chemotherapy, including a taxane, were enrolled in this single-arm, multicentre, phase 2 study in the Netherlands. The participants received trifluridine-tipiracil 35 mg/m2 orally twice a day on days 1-5 and days 8-12 during a 28-day cycle until disease progression, unacceptable toxicity, or withdrawal of consent. The primary endpoint was the disease control rate (DCR) at 8 weeks, defined as the percentage of patients that had stable disease, partial response or complete response according to RECIST 1.1, in all patients that received at least one dose of trifluridine-tipiracil and met the key eligibility criteria defined a priori. Secondary endpoints included progression-free survival (PFS), overall survival (OS), safety, and quality of life and were performed in all patients that received at least one dose of trifluridine-tipiracil. The primary endpoint was considered met, justifying further research of this treatment regimen, if the lower boundary of the 80% confidence interval (CI) exceeded 30%. The study was registered within ClinicalTrials.gov (NCT04489173) and is closed for inclusion.
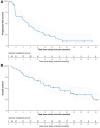
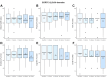
Findings: Fifty female patients were enrolled from September 2020 to July 2023, with a median of 3 (IQR, 2-3) previous endocrine therapy lines and 2 (IQR, 2-3) chemotherapy lines for MBC. The DCR rate at 8 weeks was 64.0% (n = 32, 95% CI: 50.1-75.9%; 80% CI: 55.0-72.1%), thereby meeting the primary endpoint of this study. At data cutoff (January 8, 2024), the median follow-up time was 18.2 months (IQR, 13.1-25.1 months). The median PFS was 5.4 months (95% CI: 2.0-7.2 months) and the median OS 14.0 months (95% CI: 8.8-17.8 months). The safety profile of trifluridine-tipiracil aligned with expected toxicities and included leukopenia (n = 36, 69%), neutropenia (n = 43, 83%), and fatigue (n = 43, 83%). The most common grade 3-4 AEs were primarily haematological disorders and included neutropenia (n = 38, 73%), leukopenia (n = 15, 29%) and anaemia (n = 6, 12%). The most common SAEs (any grade) with a possible relationship with trifluridine-tipiracil included anaemia (n = 2) and vomiting (n = 2). No treatment-related deaths occurred. Quality of life scores remained stable throughout the treatment.
Interpretation: Trifluridine-tipiracil demonstrated promising efficacy in heavily pre-treated patients with MBC, despite prior exposure to a fluoropyrimidine. Clinically, this suggests that trifluridine-tipiracil holds potential as a viable oral later-line treatment option with a manageable toxicity profile while maintaining quality of life. Preparations for a phase 3 trial are underway.
Funding: Servier, France.
Keywords: Chemotherapy; Metastatic breast cancer; Treatment; Trifluridine-tipiracil.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
RM reports institutional grants for investigator-initiated trials from Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Deuter Oncology, Echo Pharmaceuticals, Nordic Pharma, Novartis, Pamgene, Pfizer, Roche, Sanofi, and Servier. SvdB reports institutional grants from Servier. All remaining authors have declared no conflicts of interest.
Figures
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Rugo H.S., Rumble R.B., Macrae E., et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol. 2016;34:3069–3103. - PubMed
-
- Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. - PubMed
-
- Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

