Alkynyl Radicals, Myths and Realities
- PMID: 40017740
- PMCID: PMC11862951
- DOI: 10.1021/jacsau.4c01040
Alkynyl Radicals, Myths and Realities
Abstract
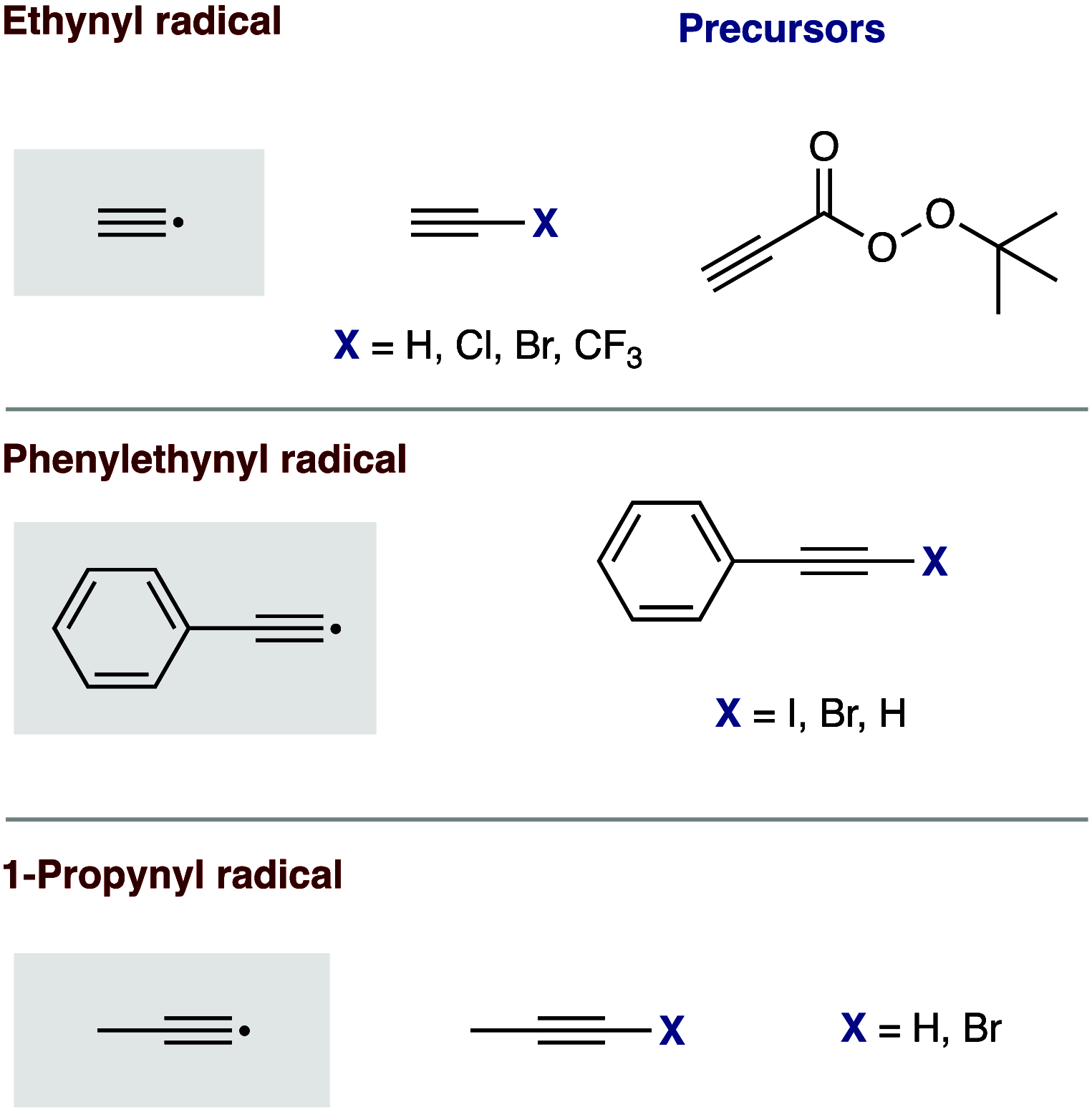
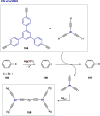
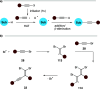
This Perspective deals with the organic chemistry of alkynyl radicals, a species that is ultimately still little known in the synthetic community. Starting with the first observations and characterizations of alkynyl radicals generated by various methodologies in the gas phase, we then particularly turned our attention to the implications of these highly reactive intermediates in organic synthesis and materials science. Mechanistic considerations have been provided, in particular, for the key steps of generating alkynyl radicals, which are mainly based on photochemical or thermal activation and single electron transfer processes. This Perspective should serve as a roadmap for the synthetic chemist in order to plan more reliably alkynylation reactions based on alkynyl radicals.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







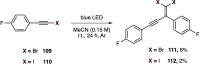
References
-
- Diederich F.; Stang P. J.; Tykwinski R. R.. Acetylene Chemistry: Chemistry, Biology and Material Science; John Wiley & Sons, 2006.
-
- Binder W. H.; Kluger C. Azide/Alkyne-“Click” Reactions: Applications in Material Science and Organic Synthesis. Curr. Org. Chem. 2006, 10 (14), 1791–1815. 10.2174/138527206778249838. - DOI
-
- Sonogashira K.; Tohda Y.; Hagihara N. A Convenient Synthesis of Acetylenes: Catalytic Substitutions of Acetylenic Hydrogen with Bromoalkenes. Iodoarenes and Bromopyridines. Tetrahedron Lett. 1975, 16 (50), 4467–4470. 10.1016/S0040-4039(00)91094-3. - DOI
Publication types
LinkOut - more resources
Full Text Sources
