Dearomative Mislow-Braverman-Evans Rearrangement of Aryl Sulfoxides
- PMID: 40017778
- PMCID: PMC11863165
- DOI: 10.1021/jacsau.4c01238
Dearomative Mislow-Braverman-Evans Rearrangement of Aryl Sulfoxides
Abstract
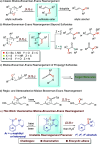
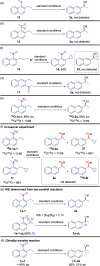
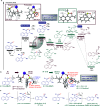
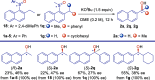
The Mislow-Braverman-Evans rearrangement, the reversible [2,3]-sigmatropic rearrangement of allylic sulfoxides to allylic sulfenate esters, finds widespread applications in organic synthesis and medicinal chemistry. However, the products of this powerful strategy have primarily been limited to derivatives of allylic alcohols. In contrast, access to structurally similar benzylic alcohols has not yet been established. Described herein is an unprecedented dearomative Mislow-Braverman-Evans rearrangement of aryl sulfoxides to afford benzylic alcohols. A variety of heteroaryl sulfoxides as well as α-naphthyl sulfoxides could be tolerated, and a diverse range of primary, secondary, and tertiary alcohols possessing either alkyl or aryl substituents can be prepared by our protocol with broad functional group tolerance. A patented bioactive molecule could be prepared using our protocol as the key step with exclusive diastereoselectivity, highlighting its potential utility in organic synthesis. Key to the success of the transformation is the dearomative tautomerization to shift the reactive alkene to the exocyclic position enabled by the reversible deprotonation of the benzylic C-H bond, setting the stage for the subsequent [2,3]-sigmatropic rearrangement. Density functional theory (DFT) calculations reveal that protonation of the α-carbon of the sulfoxide is the stereocontrolling step, generating the intermediate that undergoes [2,3]-sigmatropic rearrangement. The full reaction profile is outlined, showing the reversible nature of each step, which causes the observed erosion of the enantiopurity.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
-
For pioneering reports, see:
- Rayner D. R.; Miller E. G.; Bickart P.; Gordon A. J.; Mislow K. Mechanisms of thermal racemization of sulfoxides. J. Am. Chem. Soc. 1966, 88, 3138–3139. 10.1021/ja00965a048. - DOI
- Braverman S.; Stabinsky Y. The rearrangement of allylic trichloromethane sulphenates. Chem. Commun. 1967, 270–271. 10.1039/C19670000270. - DOI
- Bickart P.; Carson F. W.; Jacobus J.; Miller E. G.; Mislow K. Thermal racemization of allylic sulfoxides and interconversion of allylic sulfoxides and sulfenates. Mechanism and stereochemistry. J. Am. Chem. Soc. 1968, 90, 4869–4876. 10.1021/ja01020a021. - DOI
- Evans D. A.; Andrews G. C.; Sims C. L. Reversible 1,3 transposition of sulfoxide and alcohol functions. Potential synthetic utility. J. Am. Chem. Soc. 1971, 93, 4956–4957. 10.1021/ja00748a075. - DOI
-
For recent reviews, see:
- Colomer I.; Velado M.; Fernández de la Pradilla R.; Viso A. From allylic sulfoxides to allylic sulfenates: Fifty years of a never-ending [2,3]-sigmatropic rearrangement. Chem. Rev. 2017, 117, 14201–14243. 10.1021/acs.chemrev.7b00428. - DOI - PubMed
- Kaiser D.; Klose I.; Oost R.; Neuhaus J.; Maulide N. Bond-forming and -breaking reactions at sulfur(IV): Sulfoxides, sulfonium salts, sulfur ylides, and sulfinate salts. Chem. Rev. 2019, 119, 8701–8780. 10.1021/acs.chemrev.9b00111. - DOI - PMC - PubMed
-
-
-
Recently reports including chirality transfer from chiral sulfoxides, see:
- Kaldre D.; Maryasin B.; Kaiser D.; Gajsek O.; González L.; Maulide N. An asymmetric redox arylation: chirality transfer from sulfur to carbon through a sulfonium [3,3]-sigmatropic rearrangement. Angew. Chem., Int. Ed. 2017, 56, 2212–2215. 10.1002/anie.201610105. - DOI - PubMed
- Colomer I.; Ureña M.; Viso A.; Fernández de la Pradilla R. Sulfinyl-mediated stereoselective functionalization of acyclic conjugated dienes. Chem.—Eur. J. 2020, 26, 4620–4632. 10.1002/chem.201905742. - DOI - PubMed
-
-
-
For leading examples, see:
- Charest M. G.; Lerner C. D.; Brubaker J. D.; Siegel D. R.; Myers A. G. A convergent enantioselective route to structurally diverse 6-deoxytetracycline antibiotics. Science 2005, 308, 395–398. 10.1126/science.1109755. - DOI - PubMed
- Walker J. R.; Merit J. E.; Thomas-Tran R.; Tang D. T. Y.; Du Bois J. Divergent synthesis of natural derivatives of (+)-saxitoxin including 11-saxitoxinethanoic acid. Angew. Chem., Int. Ed. 2019, 58, 1689–1693. 10.1002/anie.201811717. - DOI - PMC - PubMed
-
-
- Hama N.; Matsuda T.; Sato T.; Chida N. Total synthesis of (−)-agelastatin A: the application of a sequential sigmatropic rearrangement. Org. Lett. 2009, 11, 2687–2690. 10.1021/ol900799e. - DOI - PubMed
- Bernoud E.; Le Duc G.; Bantreil X.; Prestat G.; Madec D.; Poli G. Aryl sulfoxides from allyl sulfoxides via [2,3]-sigmatropic rearrangement and domino Pd-catalyzed generation/arylation of sulfenate anions. Org. Lett. 2010, 12, 320–323. 10.1021/ol902620t. - DOI - PubMed
- Fernández de la Pradilla R.; Colomer I.; Ureña M.; Viso A. Enantiopure 1,4-diols and 1,4-aminoalcohols via stereoselective acyclic sulfoxide–sulfenate rearrangement. Org. Lett. 2011, 13, 2468–2471. 10.1021/ol200718y. - DOI - PubMed
- Kleimark J.; Prestat G.; Poli G.; Norrby P. O. Palladium-catalyzed allylic sulfinylation and the Mislow-Braverman-Evans rearrangement. Chem.—Eur. J. 2011, 17, 13963–13965. 10.1002/chem.201102937. - DOI - PubMed
- Simal C.; Bates R. H.; Ureña M.; Giménez I.; Koutsou C.; Infantes L.; Fernández de la Pradilla R.; Viso A. Synthesis of enantiopure 3-hydroxypiperidines from sulfinyl dienyl amines by diastereoselective intramolecular cyclization and [2,3]-sigmatropic rearrangement. J. Org. Chem. 2015, 80, 7674–7692. 10.1021/acs.joc.5b01307. - DOI - PubMed
- Zhang G.; Cramer N. Reductive asymmetric aza-Mislow-Evans rearrangement by 1,3,2-diazaphospholene catalysis. Angew. Chem., Int. Ed. 2023, 62, e20230107610.1002/anie.202301076. - DOI - PubMed
- Liu T.-F.; Yao Y.; Lu C.-D. Enantioselective formation of α-amino acid derivatives via [2,3]-sigmatropic rearrangement of N-acyl iminosulfinamides. Org. Lett. 2023, 25, 4156–4161. 10.1021/acs.orglett.3c01448. - DOI - PubMed
- Aouina S. M.; Lapray A.; Naubron J.-V.; Vanthuyne N.; Levacher V.; Oudeyer S.; Perrio S.; Brière J.-F. Organocatalytic strategy for a formal 1,6-conjugate hydroxylation. Org. Lett. 2024, 26, 9294–9298. 10.1021/acs.orglett.4c03482. - DOI - PubMed
-
-
For pioneering reports, see:
- Sharpless K. B.; Lauer R. F. Selenium dioxide oxidation of olefins. Evidence for the intermediacy of allylseleninic acids. J. Am. Chem. Soc. 1972, 94, 7154–7155. 10.1021/ja00775a050. - DOI
- Reich H. J. Organoselenium chemistry. Synthetic transformations based on allyl selenide anions. J. Org. Chem. 1975, 40, 2570–2572. 10.1021/jo00905a049. - DOI
- Reich H. J.; Yelm K. E. Asymmetric induction in the oxidation of [2.2]paracyclophane-substituted selenides. Application of chirality transfer in the selenoxide [2,3]-sigmatropic rearrangement. J. Org. Chem. 1991, 56, 5672–5679. 10.1021/jo00019a039. - DOI
-
For recent examples, see:
- Ohyoshi T.; Funakubo S.; Miyazawa Y.; Niida K.; Hayakawa I.; Kigoshi H. Total synthesis of (−)-13-oxyingenol and its natural derivative. Angew. Chem., Int. Ed. 2012, 51, 4972–4975. 10.1002/anie.201201383. - DOI - PubMed
- Pham D.; Basu U.; Pohorilets I.; St Croix C. M.; Watkins S. C.; Koide K. Fluorogenic probe using a Mislow-Evans rearrangement for real-time imaging of hydrogen peroxide. Angew. Chem., Int. Ed. 2020, 59, 17435–17441. 10.1002/anie.202007104. - DOI - PubMed
- Wang B.; Liu Z.; Tong Z.; Gao B.; Ding H. Asymmetric total syntheses of 8,9-seco-ent-kaurane diterpenoids enabled by an electrochemical ODI-[5 + 2] cascade. Angew. Chem., Int. Ed. 2021, 60, 14892–14896. 10.1002/anie.202104410. - DOI - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources
