Novel mRNA vaccines induce potent immunogenicity and afford protection against tuberculosis
- PMID: 40018046
- PMCID: PMC11865049
- DOI: 10.3389/fimmu.2025.1540359
Novel mRNA vaccines induce potent immunogenicity and afford protection against tuberculosis
Abstract
Introduction: Mycobacterium tuberculosis (Mtb) is the causative agent of tuberculosis (TB), a disease with a severe global burden. The intractability of Mtb has prevented the identification of clear correlates of protection against TB and hindered the development of novel TB vaccines that are urgently required. Lipid nanoparticle (LNP)-formulated mRNA is a highly promising vaccine platform that has yet to be thoroughly applied to TB.
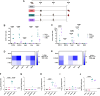
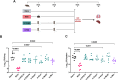
Methods: We selected five Mtb antigens (PPE15, ESAT6, EspC, EsxI, MetE) and evaluated their potential as LNP-formulated mRNA vaccines, both when each antigen was delivered individually, and when all five antigens were combined in a mix regimen (m-Mix).
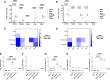
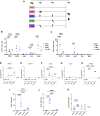
Results: Each mRNA construct demonstrated unique cellular and humoral immunogenicity, and both m-Mix, as well as the single antigen EsxI, conferred significant protection in a murine Mtb challenge model. Whilst the potent immune responses of each mRNA were maintained when applied as a boost to BCG, there was no additional increase to the efficacy of BCG. Combination of m-Mix with a recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1), in a heterologous prime-boost delivery (C-m-Mix), appeared to result in increased protection upon murine Mtb infection, than either regimen alone.
Discussion: This work warrants further investigation of LNP-formulated mRNA vaccines for TB, whilst indicating the potential of m-Mix and C-m-Mix to progress to further stages of vaccine development.
Keywords: BCG; mRNA; tuberculosis; vaccines; viral-vector.
Copyright © 2025 De Voss, Korompis, Li, Ateere, McShane and Stylianou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- World Health Organization . Global Tuberculosis Report. Geneva: World Health Organization; (2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

