Potentially commercializable nerve guidance conduits for peripheral nerve injury: Past, present, and future
- PMID: 40018056
- PMCID: PMC11867546
- DOI: 10.1016/j.mtbio.2025.101503
Potentially commercializable nerve guidance conduits for peripheral nerve injury: Past, present, and future
Abstract
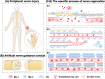
Peripheral nerve injuries are a prevalent global issue that has garnered great concern. Although autografts remain the preferred clinical approach to repair, their efficacy is hampered by factors like donor scarcity. The emergence of nerve guidance conduits as novel tissue engineering tools offers a promising alternative strategy. This review aims to interpret nerve guidance conduits and their commercialization from both clinical and laboratory perspectives. To enhance comprehension of clinical situations, this article provides a comprehensive analysis of the clinical efficacy of nerve conduits approved by the United States Food and Drug Administration. It proposes that the initial six months post-transplantation is a critical window period for evaluating their efficacy. Additionally, this study conducts a systematic discussion on the research progress of laboratory conduits, focusing on biomaterials and add-on strategies as pivotal factors for nerve regeneration, as supported by the literature analysis. The clinical conduit materials and prospective optimal materials are thoroughly discussed. The add-on strategies, together with their distinct obstacles and potentials are deeply analyzed. Based on the above evaluations, the development path and manufacturing strategy for the commercialization of nerve guidance conduits are envisioned. The critical conclusion promoting commercialization is summarized as follows: 1) The optimization of biomaterials is the fundamental means; 2) The phased application of additional strategies is the emphasized direction; 3) The additive manufacturing techniques are the necessary tools. As a result, the findings of this research provide academic and clinical practitioners with valuable insights that may facilitate future commercialization endeavors of nerve guidance conduits.
Keywords: Add-on strategy; Biomaterial; Clinical efficacy; Nerve guidance conduit; Peripheral nerve.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this manuscript.
Figures











Similar articles
-
Advances in biomaterial-based tissue engineering for peripheral nerve injury repair.Bioact Mater. 2024 Dec 13;46:150-172. doi: 10.1016/j.bioactmat.2024.12.005. eCollection 2025 Apr. Bioact Mater. 2024. PMID: 39760068 Free PMC article. Review.
-
Beyond the limiting gap length: peripheral nerve regeneration through implantable nerve guidance conduits.Biomater Sci. 2024 Mar 12;12(6):1371-1404. doi: 10.1039/d3bm01163a. Biomater Sci. 2024. PMID: 38363090 Review.
-
Dual-layer conduit containing VEGF-A - Transfected Schwann cells promotes peripheral nerve regeneration via angiogenesis.Acta Biomater. 2024 May;180:323-336. doi: 10.1016/j.actbio.2024.03.029. Epub 2024 Mar 30. Acta Biomater. 2024. PMID: 38561075
-
Additive manufacturing of peripheral nerve conduits - Fabrication methods, design considerations and clinical challenges.SLAS Technol. 2023 Jun;28(3):102-126. doi: 10.1016/j.slast.2023.03.006. Epub 2023 Apr 5. SLAS Technol. 2023. PMID: 37028493 Review.
-
In vitro efficacy of a gene-activated nerve guidance conduit incorporating non-viral PEI-pDNA nanoparticles carrying genes encoding for NGF, GDNF and c-Jun.Acta Biomater. 2018 Jul 15;75:115-128. doi: 10.1016/j.actbio.2018.06.014. Epub 2018 Jun 7. Acta Biomater. 2018. PMID: 29885855
Cited by
-
Advanced Bioactive Polymers and Materials for Nerve Repair: Strategies and Mechanistic Insights.J Funct Biomater. 2025 Jul 9;16(7):255. doi: 10.3390/jfb16070255. J Funct Biomater. 2025. PMID: 40710469 Free PMC article. Review.
-
Long-Gap Sciatic Nerve Regeneration Using 3D-Printed Nerve Conduits with Controlled FGF-2 Release.ACS Appl Mater Interfaces. 2025 Jul 16;17(28):40237-40257. doi: 10.1021/acsami.5c08237. Epub 2025 Jul 7. ACS Appl Mater Interfaces. 2025. PMID: 40621679 Free PMC article.
-
Multidimensional advances in neural interface technology for peripheral nerve repair: From material innovation to clinical translation.Mater Today Bio. 2025 Jul 14;34:102092. doi: 10.1016/j.mtbio.2025.102092. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40727303 Free PMC article. Review.
References
-
- Soto P.A., Vence M., Piñero G.M., Coral D.F., Usach V., Muraca D., Cueto A., Roig A., van Raap M.B.F., Setton-Avruj C.P. Sciatic nerve regeneration after traumatic injury using magnetic targeted adipose-derived mesenchymal stem cells. Acta Biomater. 2021;130:234–247. doi: 10.1016/j.actbio.2021.05.050. - DOI - PubMed
-
- Li J., Yao Y., Wang Y., Xu J., Zhao D., Liu M., Shi S., Lin Y. Modulation of the crosstalk between schwann cells and macrophages for nerve regeneration: a therapeutic strategy based on a multifunctional tetrahedral framework nucleic acids system. Adv. Mater. 2022;34(46) doi: 10.1002/adma.202202513. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

