Promoting π-Facial Interactions in Phenyl-Substituted 1,8-Bis(silylamido)naphthalene Alkaline Earth Complexes
- PMID: 40018372
- PMCID: PMC11863540
- DOI: 10.1021/acs.organomet.4c00479
Promoting π-Facial Interactions in Phenyl-Substituted 1,8-Bis(silylamido)naphthalene Alkaline Earth Complexes
Abstract
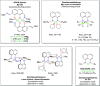
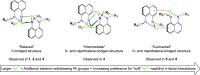
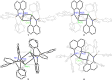
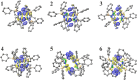
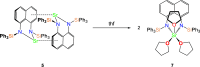
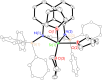
Bimetallic 1,8-bis(silylamido)naphthalene alkaline earth complexes [(R3 L)Ae]2 ([R3 L]2- = [1,8-{(R3Si)N}2C10H6)]2-, where R3 = Ph2Me, Ae = Ca (1), Sr (2), and Ba (3); R3 = Ph3, Ae = Ca (4), Sr (5), and Ba (6) were prepared via protonolysis reactions of the phenyl-substituted proligands Ph3 LH2 and Ph2MeLH2 with [AeN″2]2 (N″ = [N(SiMe3)2]-) in benzene. X-ray crystallographic analysis showed that 1, 2, and 4 crystallize as nitrogen-bridged dimers. Conversely, 5 and 6 display a naphthalene-bridged motif, while the structure of 3 is intermediate between the two distinct classes. NMR spectroscopic analysis of isolated samples of 1-6 in thf-d 8 confirmed their conversion into the monomeric thf-d 8 adducts [(R3 L)Ae(thf-d 8) n ]; crystallographic verification of the structural motif was provided by the X-ray crystal structure of [(Ph3 L)Sr(thf)3] (7). The structural range of dimers 1-6 was influenced by the electron-withdrawing nature of the phenyl substituents of the ligand and the ability to form "soft" multihaptic π-facial interactions with the metal ions, which was preferential for the larger Sr2+ and Ba2+ cations as well as the relative strength of the metal-N bonds. This has been rationalized through complementary computational studies. This work provides insight into the structure and bonding preferences of heavy alkaline earth complexes with rigid bis(amido) ligands.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Bhoelan B. S.; Stevering C. H.; van der Boog A. T.; van der Heyden M. A. Barium toxicity and the role of the potassium inward rectifier current. Clin. Toxicol. 2014, 52 (6), 584–593. 10.3109/15563650.2014.923903. - DOI - PubMed
- Hans Wedepohl K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59 (7), 1217–1232. 10.1016/0016-7037(95)00038-2. - DOI
-
- Chapple P. M.; Sarazin Y. Contemporary Molecular Barium Chemistry. Eur. J. Inorg. Chem. 2020, 2020 (35), 3321–3346. 10.1002/ejic.202000501. - DOI
- Crimmin M. R.; Hill M. S.. Homogeneous Catalysis with Organometallic Complexes of Group 2. In Alkaline-Earth Metal Compounds: Oddities and Applications; Harder S., Ed.; Springer: Berlin, Heidelberg, 2013; pp 191–241.
- Hill M. S.; Liptrot D. J.; Weetman C. Alkaline earths as main group reagents in molecular catalysis. Chem. Soc. Rev. 2016, 45 (4), 972–988. 10.1039/C5CS00880H. - DOI - PubMed
-
- Moxey G. J.; Blake A. J.; Lewis W.; Kays D. L. Alkaline Earth Complexes of a Sterically Demanding Guanidinate Ligand. Eur. J. Inorg. Chem. 2015, 2015 (36), 5892–5902. 10.1002/ejic.201501239. - DOI
- Kennedy D. B.; Evans M. J.; Jones D. D. L.; Parr J. M.; Hill M. S.; Jones C. A series of neutral alkaline earth metal hydride complexes supported by a bulky, unsymmetrical β-diketiminate ligand, [{(Dip/TCHPNacnac)M(μ-H)}2] (M = Mg, Ca, Sr or Ba). Chem. Commun. 2024, 60 (78), 10894–10897. 10.1039/D4CC04286G. - DOI - PubMed
- Harder S. Homoleptic β-Diketiminato Complexes of the Alkaline-Earth Metals: Trends in the Series Mg, Ca, Sr, and Ba. Organometallics 2002, 21 (18), 3782–3787. 10.1021/om0202827. - DOI
-
- Gao J.; Liu Y.; Zhao Y.; Yang X.-J.; Sui Y. Syntheses and Structures of Magnesium Complexes with Reduced α-Diimine Ligands. Organometallics 2011, 30 (22), 6071–6077. 10.1021/om2003199. - DOI
- Gao B.; Zhao D.; Li X.; Cui Y.; Duan R.; Pang X. Magnesium complexes bearing N,N-bidentate phenanthrene derivatives for the stereoselective ring-opening polymerization of rac-lactides. RSC Adv. 2015, 5 (1), 440–447. 10.1039/C4RA12548G. - DOI
- Ma M.; Wang H.; Wang J.; Shen L.; Zhao Y.; Xu W.-H.; Wu B.; Yang X.-J. Mg–Mg-bonded compounds with N,N′-dipp-substituted phenanthrene-diamido and o-phenylene-diamino ligands. Dalton Trans. 2019, 48 (7), 2295–2299. 10.1039/C9DT00028C. - DOI - PubMed
- Panda T. K.; Kaneko H.; Michel O.; Pal K.; Tsurugi H.; Törnroos K. W.; Anwander R.; Mashima K. Dianion and Monoanion Ligation of 1,4-Diaza-1,3-butadiene to Barium, Strontium, and Calcium. Organometallics 2012, 31 (8), 3178–3184. 10.1021/om300054h. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
