TUXEDO-4: phase II study of trastuzumab-deruxtecan in HER2-low breast cancer with new or progressing brain metastases
- PMID: 40018758
- PMCID: PMC11988270
- DOI: 10.1080/14796694.2025.2470604
TUXEDO-4: phase II study of trastuzumab-deruxtecan in HER2-low breast cancer with new or progressing brain metastases
Abstract
NCT06048718 (clinicaltrials.gov); 2023 -506,702-39-00 (EudraCT number).
Keywords: Breast; brain/neurologic; clinical trials; metastasis; novel therapy.
Plain language summary
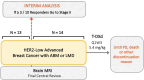
Breast cancer cells often spread to the brain (brain metastases) or the membranes surrounding the brain and spinal cord (leptomeningeal disease). Therapies to treat these serious conditions are limited. Trastuzumab deruxtecan (T-DXd), which targets a protein called HER2 (found on the surface of breast cells to control their growth) and includes a powerful anti-cancer component to kill the tumor cells, has shown clinically meaningful results in both HER2-positive (high concentration of HER2 proteins) and HER2-low (low concentration of HER2 proteins) breast cancers. Interestingly, T-DXd has successfully worked in HER2-positive breast cancer patients with brain metastases and/or leptomeningeal disease, but results in their HER2-low counterparts are limited. TUXEDO-4 is a medical study to evaluate the effectiveness of T-DXd in HER2-low breast cancer patients with recently diagnosed or worsening brain metastases, with or without aggressive leptomeningeal disease. The study is taking place in Austria and Spain, and will recruit 27 adult patients. TUXEDO-4 trial TUXEDO-4 is an international, multicenter, single-arm, two-stage optimal Simon’s design, phase II clinical trial evaluating the efficacy of trastuzumab-deruxtecan (T-DXd) in human epidermal growth factor receptor 2 (HER2)-low breast cancer (BC) patients presenting with newly diagnosed or progressing brain metastases (BMs), with or without type II leptomeningeal disease (LMD).
Conflict of interest statement
Maximilian Marhold has received honoraria for lectures, advisory board participation and consultation from Roche, Eli Lilly, Novartis, AstraZeneca, Daiichi Sankyo, Pfizer, MSD, Gilead, and Medmedia; and travel support from Amgen, Gilead, Roche, Novartis, Pierre Fabre, Daiichi Sankyo, and Eisai.
Marta Vaz-Batista has received honoraria from Daichii Sankyo, GSK, and AstraZeneca; consulting or advisory role from Daichii Sankyo and AstraZeneca; speakers’ bureau from Novartis; and travel, accomodation, and expenses from AstraZeneca.
Isabel Blancas has received grants and research support to the Institution from AstraZeneca, Lilly, Pfizer and Roche; honoraria and advisor collaboration from AstraZeneca, Roche, Novartis, Eisai, Celgene, Pfizer, Lilly, Pierre–Fabre, Bristol–Myers Squibb, Daiichi Sankyo, Grünenthal, Seagen, and Veracyte; and support for attending meetings and/or travel from AstraZeneca, Roche, Novartis, Pfizer, Lilly, Pierre-Fabre, Bristol-Myers Squibb, and Daiichi Sankyo.
Cristina Saura-Manich has received research support and consulting or advisory role from Genentech, AstraZeneca, Aragon Pharmaceuticals, Bayer Pharma, Byondis, Boehringer Ingelheim, Bristol-Myers Squibb, Cytomx Therapeutics, Daiichi Sankyo, F. Hoffmann-La Roche, Eisai, Genomic Health, GlaxoSmithKline, Innoup Farma, Eli Lilly, Macrogenics, Menarini Ricerche, Merck Sharp & Dohme, Merus, Millennium Pharmaceuticals, Novartis, Pfizer, Pierre Fabre, PintPharma, Puma Biotechnology, Sanofi, Seattle Genetics, and Zymeworks.
Manuel Ruíz-Borrego has received consulting fees from Roche and Puma, and honoraria expenses from Roche/Genentech, Pfizer, and Novartis.
Felipe Slebe, Marta Campolier, Juliana Carvalho-Santos, José Antonio Guerrero-Martínez, and Carlos Jiménez-Cortegana declare to be full-time employees at MEDSIR.
Rupert Bartsch has received honoraria from AstraZeneca, BMS, Daiichi-Sankyo, Eisai, and Eli-Lilly; consulting or advisory role from AstraZeneca, Daichi, Eisai, and Eli-Lilly; research funding from Daiichi-Sankyo; and travel, accommodations, and expenses from MSD, Daiichi-Sankyo, Novartis, and Pfizer.
Matthias Preusser has received honoraria for lectures, consultation and advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, Janssen, Servier, Miltenyi, Böhringer-Ingelheim, Telix, Medscape, OncLive.
Cristina Morales, Cristina Saavedra, Patricia Cortez, Beate Rottenmanner, and Heidrun Forstner have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- Hofer S, Le Rhun E. Leptomeningeal metastases from solid tumours. Memo-Mag Eur Med Oncol. 2021;14(2):192–197. doi:10.1007/s12254-021-00693-6 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
