Dexmedetomidine to Reduce Vasopressor Resistance in Refractory Septic Shock: α2 Agonist Dexmedetomidine for REfractory Septic Shock (ADRESS): A Double-Blind Randomized Controlled Pilot Trial
- PMID: 40019329
- PMCID: PMC11952692
- DOI: 10.1097/CCM.0000000000006608
Dexmedetomidine to Reduce Vasopressor Resistance in Refractory Septic Shock: α2 Agonist Dexmedetomidine for REfractory Septic Shock (ADRESS): A Double-Blind Randomized Controlled Pilot Trial
Abstract
Objectives: Increasing evidence has suggested the benefits of dexmedetomidine in patients with sepsis. Dexmedetomidine may increase vasopressor sensitivity, which may be of interest in the setting of refractory septic shock. The α2 Agonist Dexmedetomidine for REfractory Septic Shock (ADRESS) pilot study aimed to evaluate the effect of dexmedetomidine on the vasopressor response in patients with refractory septic shock.
Design: This study was a multicenter, randomized, placebo-controlled, double-blind pilot trial.
Setting: The study was conducted in 5 ICUs in France.
Patients: Inclusion criteria were septic shock (Sepsis-3 definition) and norepinephrine requirement greater than or equal to 0.25 µg/kg/min (0.5 µg/kg/min of norepinephrine tartrate) with persistent circulatory failure (defined by lactate > 2 mmol/L, oliguria, or skin mottling) and invasive mechanical ventilation.
Interventions: The arterial pressure response to phenylephrine was measured before starting the treatment (0 hr), at 6 hours (primary outcome), and 12 hours. In the treatment arm, dexmedetomidine was given at a fixed dose of 1 µg/kg/hr.
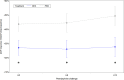
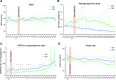
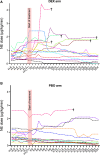
Measurements and main results: Inclusions were stopped early because of higher mortality in the dexmedetomidine arm. Thirty-two patients of the 36 planned were included. Response to phenylephrine at 6 hours was lower in the dexmedetomidine group than in the placebo group (1.26 ± 0.23 vs. 1.45 ± 0.26; p = 0.048), although this difference was also observed at baseline ( p = 0.029). There were no significant differences between the groups in terms of cumulative norepinephrine dose, lactatemia, Sequential Organ Failure Assessment score, fluid balance, ventilation-free days, or occurrence of bradycardia. Mortality on day 3 was higher in the dexmedetomidine group than in the placebo group, with a difference that diminished and was no longer significant on 30 and 90 days.
Conclusions: Patients in the dexmedetomidine arm had a significantly lower response to phenylephrine at all study times including baseline, which might have contributed to higher early mortality in the dexmedetomidine arm and preclude to conclude on dexmedetomidine efficacy in refractory septic shock. However, heart rate was not decreased in the dexmedetomidine arm.
Trial registration: ClinicalTrials.gov NCT03953677.
Keywords: alpha-2 agonists; dexmedetomidine; refractory septic shock; septic shock; vasopressors.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr. Dargent received support for article research from the GIRCI grant. Drs. Dargent and Cransac disclosed off-label use of dexemedtomidine. Dr. Cransac received funding from Fondation Clément-Drevon. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
-
- Dargent A, Nguyen M, Fournel I, et al. : Vasopressor cumulative dose requirement and risk of early death during septic shock: An analysis from the EPISS cohort. Shock. 2017; 49:625–630 - PubMed
-
- Avontuur JA, Nolthenius RPT, Buijk SL, et al. : Effect of L-NAME, an inhibitor of nitric oxide synthesis, on cardiopulmonary function in human septic shock. Chest. 1998; 113:1640–1646 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

