MISP Suppresses Ferroptosis via MST1/2 Kinases to Facilitate YAP Activation in Non-Small Cell Lung Cancer
- PMID: 40019375
- PMCID: PMC12021056
- DOI: 10.1002/advs.202415814
MISP Suppresses Ferroptosis via MST1/2 Kinases to Facilitate YAP Activation in Non-Small Cell Lung Cancer
Abstract
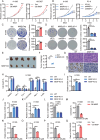
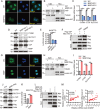
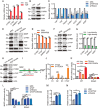
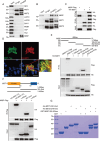
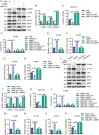
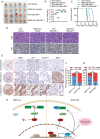
Despite advances in non-small cell lung cancer (NSCLC) therapies, resistance remains a major challenge. Ferroptosis, a form of regulated cell death, plays a key role in cancer progression and treatment response. However, the mechanisms governing ferroptosis in NSCLC are not fully understood. The Hippo pathway, which regulates cell proliferation, has recently been implicated in ferroptosis regulation. In this study, we identify Mitotic Spindle Positioning (MISP) as a critical inhibitor of ferroptosis in NSCLC. MISP is upregulated in NSCLC tissues, and its loss sensitizes cells to ferroptosis, reducing cell proliferation in vitro and in vivo. Mechanistically, MISP binds to the SARAH domain of MST1/2 kinases, inhibiting their homodimerization and autophosphorylation, leading to sustained activation of YAP, a transcriptional coactivator in the Hippo pathway. YAP activation increases SLC7A11 expression, which protects cells from ferroptosis. We also identify a mutant MISP-R390/391A that disrupts MISP-MST1/2 binding, further illustrating the MST1/2-dependent inhibition of Hippo signaling. Notably, MISP is a target of YAP, creating a feedback loop that amplifies YAP signaling. Our findings suggest a novel MISP-YAP axis regulating ferroptosis, positioning MISP as a potential therapeutic target for NSCLC, especially in cases with dysregulated YAP.
Keywords: MISP; MST kinases; SLC7A11; YAP; ferroptosis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
