SENP3 Drives Abdominal Aortic Aneurysm Development by Regulating Ferroptosis via De-SUMOylation of CTH
- PMID: 40019399
- PMCID: PMC12021093
- DOI: 10.1002/advs.202414500
SENP3 Drives Abdominal Aortic Aneurysm Development by Regulating Ferroptosis via De-SUMOylation of CTH
Abstract
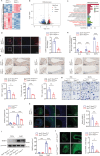
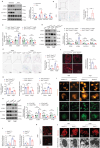
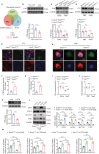
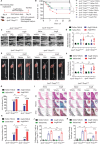
Abdominal aortic aneurysm (AAA) is a high-risk inflammatory disorder. SENP3, a SUMO2/3-specific protease, is closely involved in the development of cancer. In this study, the aim is to explore the role of SENP3 in macrophages in AAA. It is found that the protein expression of SENP3 is significantly upregulated in both human and murine AAA specimens. SENP3 expression is negatively regulated by the E3 ubiquitin ligase STUB1/CHIP. Furthermore, myeloid-specific SENP3 knockout inhibited AAA formation in both AngII- and CaCl2-induced mouse models. SENP3 deficiency repressed AAA lesion macrophage infiltration and inflammatory response. Mechanistic studies identified Cystathionine Gamma-Lyase (CTH), a critical enzyme involved in hydrogen sulfide production, as a target protein of SENP3 that mediated the exacerbating effects of SENP3 on ferroptosis and inflammatory programs in macrophages. SUMO-3 modification at Lysine 361 promoted CTH protein stability, whereas de-SUMOylation by SENP3 facilitated its proteasome-dependent degradation. Most importantly, it is found that CTH inhibitor counteracted the protective effect of SENP3 deficiency on AAA. Additionally, supplementation with ATB346, a novel H2S-donating naproxen derivative, prevented AAA development in mice. These studies suggest that SENP3-mediated CTH deSUMOylation regulates macrophage ferroptosis and AAA development. The SENP3/CTH axis is therefore an important therapeutic target for aortic aneurysmal diseases.
Keywords: CTH; SENP3; SUMOylation; abdominal aortic aneurysm; ferroptosis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Raffort J., Lareyre F., Clément M., Hassen‐Khodja R., Chinetti G., Mallat Z., Nat. Rev. Cardiol. 2017, 14, 457. - PubMed
MeSH terms
Substances
Grants and funding
- 82130012/National Natural Science Foundation of China
- 82300489/National Natural Science Foundation of China
- 81830010/National Natural Science Foundation of China
- 81870338/National Natural Science Foundation of China
- 82100447/National Natural Science Foundation of China
- 23PJD084/Shanghai Pujiang Program
- 2022YNJCQ03/Nurture projects for basic research of Shanghai Chest Hospital
- SHDC2020CR1029B/Clinical Research Plan of SHDC
- SHSMU-ZLCX20212302/Innovative Research Team of High-level Local Universities
- 24ZR1464500/Shanghai Action Plan for Science, Technology and Innovation
LinkOut - more resources
Full Text Sources
