An image-computable model of speeded decision-making
- PMID: 40019474
- PMCID: PMC11870652
- DOI: 10.7554/eLife.98351
An image-computable model of speeded decision-making
Abstract
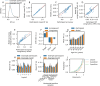
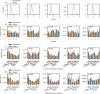
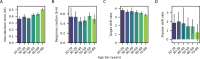
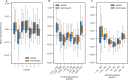
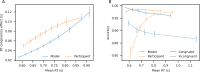
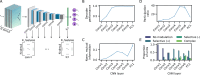
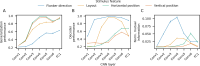
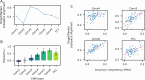
Evidence accumulation models (EAMs) are the dominant framework for modeling response time (RT) data from speeded decision-making tasks. While providing a good quantitative description of RT data in terms of abstract perceptual representations, EAMs do not explain how the visual system extracts these representations in the first place. To address this limitation, we introduce the visual accumulator model (VAM), in which convolutional neural network models of visual processing and traditional EAMs are jointly fitted to trial-level RTs and raw (pixel-space) visual stimuli from individual subjects in a unified Bayesian framework. Models fitted to large-scale cognitive training data from a stylized flanker task captured individual differences in congruency effects, RTs, and accuracy. We find evidence that the selection of task-relevant information occurs through the orthogonalization of relevant and irrelevant representations, demonstrating how our framework can be used to relate visual representations to behavioral outputs. Together, our work provides a probabilistic framework for both constraining neural network models of vision with behavioral data and studying how the visual system extracts representations that guide decisions.
Keywords: decision-making; neural network modelling; neuroscience; none; visual processing.
© 2024, Jaffe et al.
Conflict of interest statement
PJ, GS, RS, PB, RP No competing interests declared
Figures




Update of
- doi: 10.48550/arXiv.2403.16382
- doi: 10.7554/eLife.98351.1
- doi: 10.7554/eLife.98351.2
References
-
- Ansuini A, Laio A, H.Macke J, Zoccolan D. Intrinsic Dimension of Data Representations in Deep Neural Networks. Adv Neural Inf Process Systems; 2019.
MeSH terms
LinkOut - more resources
Full Text Sources

