Impact of a minimal monitoring HCV treatment approach on Health-Related Quality of Life
- PMID: 40019678
- PMCID: PMC12413691
- DOI: 10.1007/s11136-025-03922-1
Impact of a minimal monitoring HCV treatment approach on Health-Related Quality of Life
Abstract
Background: Direct-acting antivirals (DAA) are highly effective for the management of HCV disease. This study aims to evaluate changes in health-related quality of life (HQoL) among people with HCV who were treated with DAAs using a minimal monitoring (MINMON) approach.
Methods: ACTG A5360 was a multicenter, international (Brazil, South Africa, Thailand, Uganda, and USA) trial to assess the feasibility and efficacy of MINMON approach in people with HCV. We measured HQoL using EQ-5D-3L at baseline, sustained virological evaluation visit, week 48 and week 72, and described using EQ-5D summary index (ranges:0-1) and visual analog scale (VAS) score (ranges 0-100). We used paired T-tests to evaluate the change in EQ-5D summary and multivariable linear regression for changes in VAS scores.
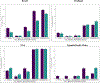
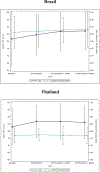
Results: Overall, 394 individuals were included; most did not have cirrhosis (360; 91%) or problematic alcohol use (278; 71%). We found HQoL improvements for participants from Brazil and Thailand, but not for the USA. Participants reported high rates of pain/discomfort and anxiety/depression, with decreases over time only for Brazil. Factors associated with larger improvements in VAS scores included: cirrhosis at baseline, and non-use or problematic use of other substances (apart from tobacco/marijuana) compared to non-problematic use.
Conclusion: We found HQoL improvements among people with HCV following DAA treatment with variability across countries. Our findings reinforce the importance of DAA treatment, especially among those with advanced HCV disease. Continuous mental health care including depression and substance use support should be offered to individuals after HCV treatment.
Trial registration number: NCT03512210 (22-Oct-2018) Direct-acting antivirals are highly effective for the management of hepatitis C disease. Recent studies have shown that hepatitis C treatment with direct-acting antivirals has a positive impact on quality of life. However, no studies have assessed quality of life among individuals starting direct-acting antivirals treatment across multiple countries representing high-, middle- and low-income settings. In this study, we evaluated changes in health-related quality of life among people with hepatitis C who were treated with direct-acting antivirals using a simplified treatment delivery and offering minimal in-person monitoring. We found quality of life improvements among persons with hepatitis C after direct-acting antivirals treatment, with differences across countries. Although direct-acting antivirals treatment works well and should be available to all persons with hepatitis C, we found that participants with cirrhosis (liver damage) had higher improvements in quality of life, reinforcing the importance of starting treatment among people with advanced hepatitis C. We also found high rates of anxiety and depression, pointing to the importance of adding mental health care and support to infectious diseases services.
Keywords: Cirrhosis; Direct-acting antivirals; HIV; Health-related quality of life; Hepatitis C; Mental health.
© 2025. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Declarations. Ethical approval: The ACTG A5360 protocol was approved by institutional review or ethical review boards of all 38 participating sites. Interim reviews for conduct and safety were performed at least yearly by a network appointed independent study monitoring committee. Consent to participate: Informed consent was obtained from all participants included in the study. Competing interests: The authors declare no conflict of interest.
Figures

References
-
- WHO. (2024, September 4). Global hepatitis report 2024: Action for access in low- and middle-income countries. https://www.who.int/publications/i/item/9789240091672
-
- Schulte B, Schmidt CS, Manthey J, Strada L, Christensen S, Cimander K, Görne H, Khaykin P, Scherbaum N, Walcher S, Mauss S, Schäfer I, Verthein U, Rehm J, & Reimer J (2020). Clinical and Patient-Reported Outcomes of Direct-Acting Antivirals for the Treatment of Chronic Hepatitis C Among Patients on Opioid Agonist Treatment: A Real-world Prospective Cohort Study. Open Forum Infectious Diseases, 7(8), ofaa317. 10.1093/ofid/ofaa317 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

