Differentiation versus dysfunction: thyroid hormone, deiodinases and retinal photoreceptors
- PMID: 40019772
- PMCID: PMC11906153
- DOI: 10.1530/ETJ-24-0315
Differentiation versus dysfunction: thyroid hormone, deiodinases and retinal photoreceptors
Abstract
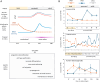
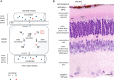
A growing body of evidence has established that thyroid hormone (triiodothyronine, T3) is a key factor in the differentiation and survival of the light-sensing photoreceptors in the retina. These functions include a critical role in generating the cone photoreceptor diversity that is required for color vision. Here, we review some of these functions of T3 and the critical mechanisms that regulate the T3 signal in the mammalian retina. The provision of T3, the active form of thyroid hormone, is determined by developmentally rising levels of T3 and its precursor T4 (thyroxine) in the circulation and by intrinsic control within the retina itself by deiodinase enzymes that deplete or amplify the available level of T3. Dynamic profiles of inactivating (DIO3) and activating (DIO2) deiodinases suggest that the T3 signal is progressively calibrated throughout early development, maturation and later functional maintenance of the retina. However, the benefits of T3 come at a cost: photoreceptors are susceptible to impairment and cell death when T3 signaling becomes imbalanced. These findings have implications regarding the influence of T3 in retinal diseases.
Keywords: color vision; deiodinase enzyme; photoreceptor; retina; thyroid hormone.
Conflict of interest statement
The authors have no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

