Optogenetic quantification of cardiac excitability and electrical coupling in intact hearts to explain cardiac arrhythmia initiation
- PMID: 40020054
- PMCID: PMC11870084
- DOI: 10.1126/sciadv.adt4103
Optogenetic quantification of cardiac excitability and electrical coupling in intact hearts to explain cardiac arrhythmia initiation
Abstract
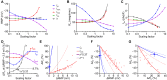
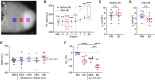
Increased cardiac excitability and reduced electrical coupling promote cardiac arrhythmia and can be quantified by input resistance (Rm), pacing threshold (Ithr), and cardiac space constant (λ). However, their measurement in the heart was not feasible because the required homogenous current injection cannot be performed with electrical stimulation. We overcame this problem by optogenetic current injection into all illuminated cardiomyocytes of mouse hearts in different action potential phases. Precisely triggered and patterned illumination enabled measuring Rm and λ, which both were smallest at diastole. Pharmacological and depolarization-induced reduction of inwardly rectifying K+ currents (IK1), gap junction block, and cardiac infarction reduced Ithr, showing the importance of high IK1 density and intact cardiomyocyte coupling for preventing arrhythmia initiation. Combining optogenetic current injection and computer simulations was used to classify pro- and anti-arrhythmic mechanisms based on their effects on Rm and Ithr and allowed to quantify IK1 inward rectification in the intact heart, identifying reduced IK1 rectification as anti-arrhythmic concept.
Figures





References
-
- Spector P., Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ. Arrhythm. Electrophysiol. 6, 655–661 (2013). - PubMed
-
- Dhamoon A. S., Jalife J., The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm. 2, 316–324 (2005). - PubMed
-
- Hegyi B., Bossuyt J., Ginsburg K. S., Mendoza L. M., Talken L., Ferrier W. T., Pogwizd S. M., Izu L. T., Chen-Izu Y., Bers D. M., Altered repolarization reserve in failing rabbit ventricular myocytes: Calcium and β-adrenergic effects on delayed- and inward-rectifier potassium currents. Circ. Arrhythm. Electrophysiol. 11, e005852 (2018). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

