Composition and function of AChR chimeric autoantibody receptor T cells for antigen-specific B cell depletion in myasthenia gravis
- PMID: 40020066
- PMCID: PMC11870065
- DOI: 10.1126/sciadv.adt0795
Composition and function of AChR chimeric autoantibody receptor T cells for antigen-specific B cell depletion in myasthenia gravis
Abstract
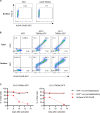
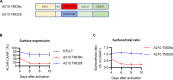
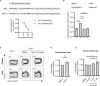
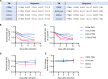
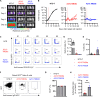
In acetylcholine receptor (AChR)-seropositive myasthenia gravis (MG), anti-AChR autoantibodies impair neuromuscular transmission and cause severe muscle weakness. MG therapies broadly suppress immune function, risking infections. We designed a chimeric autoantibody receptor (CAAR) expressing the 210-amino acid extracellular domain of the AChR α subunit (A210) linked to CD137-CD3ζ cytoplasmic domains to direct T cell cytotoxicity against anti-AChRα B cells. A210-CAART incorporating a CD8α transmembrane domain (TMD8α) showed functional but unstable surface expression, partially restored by inhibiting lysosomal degradation. A210-CAART with a CD28 TMD showed sustained surface expression, independent of TMD dimerization motifs. In a mouse xenograft model, A210.TMD8α-CAART demonstrated early control of anti-AChR B cell outgrowth but subsequent rebound and loss of surface CAAR expression, whereas A210.TMD28-CAART induced sustained surface CAAR expression and target cell elimination. This study demonstrates the importance of the CD28 TMD for CAAR stability and in vivo function, laying the groundwork for future development of precision cellular immunotherapy for AChR-MG.
Figures


References
-
- Haghikia A., Hegelmaier T., Wolleschak D., Bottcher M., Desel C., Borie D., Motte J., Schett G., Schroers R., Gold R., Mougiakakos D., Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 22, 1104–1105 (2023). - PubMed
-
- Muller F., Taubmann J., Bucci L., Wilhelm A., Bergmann C., Volkl S., Aigner M., Rothe T., Minopoulou I., Tur C., Knitza J., Kharboutli S., Kretschmann S., Vasova I., Spoerl S., Reimann H., Munoz L., Gerlach R. G., Schafer S., Grieshaber-Bouyer R., Korganow A. S., Farge-Bancel D., Mougiakakos D., Bozec A., Winkler T., Kronke G., Mackensen A., Schett G., CD19 CAR T-cell therapy in autoimmune disease–A case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024). - PubMed
-
- Motte J., Sgodzai M., Schneider-Gold C., Steckel N., Mika T., Hegelmaier T., Borie D., Haghikia A., Mougiakakos D., Schroers R., Gold R., Treatment of concomitant myasthenia gravis and Lambert-Eaton myasthenic syndrome with autologous CD19-targeted CAR T cells. Neuron 112, 1757–1763.e2 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

