Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19: an international, randomised, open-label trial
- PMID: 40020259
- PMCID: PMC11919330
- DOI: 10.1016/j.ebiom.2025.105613
Early, very high-titre convalescent plasma therapy in clinically vulnerable individuals with mild COVID-19: an international, randomised, open-label trial
Abstract
Background: COVID-19 convalescent plasma (CCP) is a treatment option for COVID-19. This study investigated the safety and efficacy of early, very high-titre CCP in immunocompromised individuals with mild COVID-19.
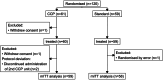
Methods: This randomised, controlled, open-label trial assessed CCP in immunocompromised patients (n = 120) with mild COVID-19 in 10 clinical trial centres across Germany, France, and the Netherlands. Patients were randomised 1:1 to receive either standard of care (SoC) alone (SoC group) or SoC and 2 units of CCP. Most patients (89.7%) had received ≥3 SARS-CoV-2 vaccinations. The primary endpoint was hospitalisation for progressive COVID-19 symptoms or death by day 28 after randomisation, analysed on a modified intention-to-treat basis (117 patients). The safety analysis included the full analysis set. The trial is registered with EudraCT 2021-006621-22, and ClinicalTrials.gov, NCT05271929.
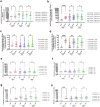
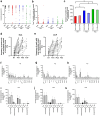
Findings: Between April 11, 2022 and November 27, 2023, 120 patients were enrolled. Patients in the CCP group received a median of 559 ml CCP from convalescent, vaccinated donors with very high levels of SARS-CoV-2 antibodies (median 81,810 IU/ml) at a median 4 days after symptom onset. The primary outcome occurred in 5/58 patients (8.6%) in the SoC group and in 0/59 patients (0%) in the CCP group, difference -8.6% (95% confidence interval of difference -19% to -0.80%; p-value 0.027; Fisher's exact test). The course of SARS-CoV-2 antibodies in the patients demonstrated a passive transfer of antibodies by the CCP, in particular neutralising effects against new SARS-CoV-2 variants. Whole genome sequencing of SARS-CoV-2 in patients during follow-up showed significant intra-host viral evolution, but without differences between groups. CCP was well tolerated.
Interpretation: Early administration of high-titre CCP can prevent hospitalisation or death in immunocompromised patients with mild COVID-19.
Funding: Support-e project (European Union's Horizon 2020 Programme), German Federal Ministry of Education and Research, ZonMw, the Netherlands Organisation for Health Research and Development.
Keywords: COVID-19; Convalescent plasma; Neutralising antibody; Randomised trial; SARS-CoV-2.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests PT is an employee of Établissement Français du Sang, the blood establishment responsible for blood collection, qualification and supply in France. HS, SH, SK, HH, MS are, and ESe was an employee of the German Red Cross Blood Transfusion Service Baden-Württemberg-Hessen (or its affiliates), the establishment responsible for blood collection, qualification, and supply of blood products (including CCP) in several federal states, Germany. LJE and DJR are employees of NHS Blood and Transplant, the blood establishment responsible for blood establishment responsible for blood collection, qualification and supply in England. KB reports grants/contracts with Alexion, Astellas, AstraZeneca, Chiesi, CSL Behring, MSC, Otsuka, Stada, Takeda (all payments to his institution); consulting fees from Aicuris, Alexion, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Carealytics, CareDx, Chiesi, CSL Behring, Fresenius, Hans, HiBio, MSD, Natera, Neovii, Paladin, Pfizer, Pirche, Sanofi, Stada, Takeda, Veloxis, Vifor, Xenothera; honoraria for lectures, presentation, speaker's bureaus manuscript writing or educational events from Astellas, AstraZeneca, Chiesi, Fresenius, Hansa, MSD, Neovii, Paladin, Sanofi, Takeda; support for attending meetings and/or travel from AstraZeneca, Chiesi, Hansa, HiBio, MSC, Neovii, Paladin, Stada, Tadeda, Veloxis; participation on a Data Safety Monitoring Board or Advisory Board for Aicuris, Alexion, Astellas, AstraZenca, Bristol-Myers-Squipp, Carealytics, CareDx, Chiesi, CSL Behring, HiBio, MSC, Natera, Neovii, Paladin, Pfizer, Stada, Takeda, Veloxis, Vifor; leadership or fiduciary role in the German Transplant Organisation and Eurotransplant. ED reports honoraria for honoraria for lectures, presentation, speaker's bureaus manuscript writing or educational events from Janssen, Jazz Pharmaceuticals (France); support for attending meetings from Novartis, Sanofi. JS reports support for attending meetings/travel from Pierre Fabre and stocks from BioNTech, Roche and Johnson & Johnson. The authors declare no other competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

