Development of an oral regimen of unithiol for the treatment of snakebite envenoming: a phase 1 open-label dose-escalation safety trial and pharmacokinetic analysis in healthy Kenyan adults
- PMID: 40020260
- PMCID: PMC11919382
- DOI: 10.1016/j.ebiom.2025.105600
Development of an oral regimen of unithiol for the treatment of snakebite envenoming: a phase 1 open-label dose-escalation safety trial and pharmacokinetic analysis in healthy Kenyan adults
Abstract
Background: Viperidae snakes are responsible for many of the 94,000 deaths caused by snakebite envenoming each year. The most pathological venom component of this globally diverse family of snakes are the zinc-dependent snake venom metalloproteinase (SVMP) enzymes, which can be inhibited by the metal chelator, unithiol. A short-course oral regimen, readily available and rapidly deployed ahead of hospital admission is needed.
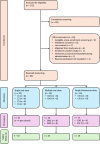
Methods: This open-label, phase 1 clinical trial assessed the safety of single ascending oral, multiple ascending oral, and single ascending intravenous doses of unithiol in 64 healthy adult volunteers from Kilifi County, Kenya. The multiple dose stage was informed by an interim safety and pharmacokinetic analysis, and predefined target plasma concentrations. Plasma concentrations of unithiol were measured using high-performance liquid chromatography-mass spectrometry, and safety was described by full adverse event reporting.
Findings: 175 individuals were screened, and 64 (median age 30 years, IQR 25-38 years) received the study drug. There were no dose limiting toxicities or serious adverse events. There were 61 solicited adverse events, 17 related unsolicited adverse events, and 53 laboratory adverse events, all of mild or moderate severity. The maximum oral dose of 1500 mg was well tolerated and associated with the following pharmacokinetic parameters: Cmax 14.7 μg/mL, Tmax 2.9 h, T1/2 18.4 h, and AUC0-∞ 204.5 μg.h/mL.
Interpretation: The phase 2 recommended dose (1500 mg loading dose, followed by 900 mg doses at 6-h and 24-h) has no safety concerns, and has promising pharmacokinetic properties for clinical use. Unithiol is affordable, stable at room temperature, and has the potential to be given orally in remote rural clinics. Its further development for snakebite indication is warranted.
Funding: Wellcome Trust, Bloomsbury Set, and Cures Within Reach.
Keywords: Envenoming; Phase 1 clinical trial; Snakebite; Unithiol.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests MA, YKN, SN, DR, JM, CM, BO, LE, AA, LD, RHC, APW, IA, FMN, RF, SK, DGL, MH all declare no conflicts of interest. LOA, JK and NRC are named inventors on a patent application relating to the use of unithiol for snakebite indication. The manufacturer (Heyl Chemisch-pharmazeutische Fabrik GmbH & Co. KG) provided the study drug without charge for the purposes of this trial. The manufacturer had no role in the study design, data analysis, or the decision to publish the results.
Figures

References
-
- Gutiérrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite envenoming. Nat Rev Dis Prim. 2017;3 - PubMed
-
- Gutiérrez J.M., León G., Rojas G., Lomonte B., Rucavado A., Chaves F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon. 1998;36:1529–1538. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

