A humanised ACE2, TMPRSS2, and FCGRT mouse model reveals the protective efficacy of anti-receptor binding domain antibodies elicited by SARS-CoV-2 hybrid immunity
- PMID: 40020261
- PMCID: PMC11910679
- DOI: 10.1016/j.ebiom.2025.105619
A humanised ACE2, TMPRSS2, and FCGRT mouse model reveals the protective efficacy of anti-receptor binding domain antibodies elicited by SARS-CoV-2 hybrid immunity
Abstract
Background: Despite the importance of vaccination- and infection-elicited antibodies (Abs) to SARS-CoV-2 immunity, current mouse models do not fully capture the dynamics of Ab-mediated immunity in vivo, including potential contributions of the neonatal Fc receptor, encoded by FCGRT.
Methods: We generated triple knock-in (TKI) mice expressing human ACE2, TMPRSS2, and FCGRT; and evaluated the protective efficacy of anti-SARS-CoV-2 monoclonal Abs (mAbs) and plasma from individuals with immunity elicited by vaccination alone plus SARS-CoV-2 infection-induced (hybrid) immunity.
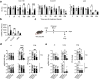
Findings: A human anti-SARS-CoV-2 mAb harbouring a half-life-extending mutation, but not the wild-type mAb, exhibited prolonged half-life in TKI mice and protected against lung infection with Omicron BA.2, validating the utility of these mice for evaluating therapeutic Abs. Pooled plasma from individuals with hybrid immunity to Delta, but not from vaccinated-only individuals, cleared infectious Delta from the lungs of TKI mice (P < 0.01), even though the two plasma pools had similar Delta-binding and -neutralising Ab titres in vitro. Similarly, plasma from individuals with hybrid Omicron BA.1/2 immunity, but not hybrid Delta immunity, decreased lung infection (P < 0.05) with BA.5 in TKI mice, despite the plasma pools having comparable BA.5-binding and -neutralising titres in vitro. Depletion of receptor-binding domain-targeting Abs from hybrid immune plasma abrogated their protection against infection.
Interpretation: These results demonstrate the utility of TKI mice as a tool for the development of anti-SARS-CoV-2 mAb therapeutics, show that in vitro neutralisation assays do not accurately predict in vivo protection, and highlight the importance of hybrid immunity for eliciting protective anti-receptor-binding domain Abs.
Funding: This work was funded by grants from the e-Asia Joint Research Program (N10A650706 and N10A660577 to MLM, in collaboration with SS); the NIH (U19 AI142790-02S1 to EOS and SS and R44 AI157900 to KJ); the GHR Foundation (to SS and EOS); the Overton family (to SS and EOS); the Arvin Gottlieb Foundation (to SS and EOS), the Prebys Foundation (to SS); and the American Association of Immunologists Fellowship Program for Career Reentry (to FASB).
Keywords: Delta; Hybrid immunity; Monoclonal antibodies; Mouse model; Omicron BA.1; Omicron BA.2; Omicron BA.5; Polyclonal antibodies; SARS-CoV-2; hACE2; hFCGRT; hTMPRSS2.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests NS, CC, DRW, and KJ are employees and stockholders of Synbal, Inc. All other authors declare no conflicts of interest.
Figures



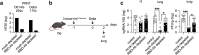
References
-
- World Health Organization Coronavirus (COVID-19) dashboard. https://data.who.int/dashboards/covid19/cases
-
- CDC's Laboratory Outreach Communication System (LOCS) 09/22/2021: lab advisory: CDC updates SARS-CoV-2 variant classifications. https://www.cdc.gov/locs/2021/09-22-2021-lab-advisory-CDC-Updates-SARS-C...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

