Cardiac differentiation roadmap for analysis of plasticity and balanced lineage commitment
- PMID: 40020683
- PMCID: PMC11960529
- DOI: 10.1016/j.stemcr.2025.102422
Cardiac differentiation roadmap for analysis of plasticity and balanced lineage commitment
Abstract
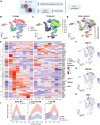
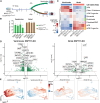
Stem cell-based models of human heart tissue and cardiac differentiation employ monolayer and 3D organoid cultures with different properties, cell type composition, and maturity. Here we show how cardiac monolayer, embryoid body, and engineered heart tissue trajectories compare in a single-cell roadmap of atrial and ventricular differentiation conditions. Using a multiomic approach and gene-regulatory network inference, we identified regulators of the epicardial, atrial, and ventricular cardiomyocyte lineages. We identified ZNF711 as a regulatory switch and safeguard for cardiomyocyte commitment. We show that ZNF711 ablation prevents cardiomyocyte differentiation in the absence of retinoic acid, causing progenitors to be diverted more prominently to epicardial and other lineages. Retinoic acid rescues this shift in lineage commitment and promotes atrial cardiomyocyte differentiation by regulation of shared and complementary target genes, showing interplay between ZNF711 and retinoic acid in cardiac lineage commitment.
Keywords: ZNF711; cardiac lineage commitment; epicardial cells; gene-regulatory networks; heart fields; human pluripotent stem cells; retinoic acid; single-cell multiomics.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.P. is a cofounder of Pluriomics (Ncardia) and River BioMedics BV.
Figures





References
-
- Ameen M., Sundaram L., Shen M., Banerjee A., Kundu S., Nair S., Shcherbina A., Gu M., Wilson K.D., Varadarajan A., et al. Integrative single-cell analysis of cardiogenesis identifies developmental trajectories and non-coding mutations in congenital heart disease. Cell. 2022;185:4937–4953.e23. doi: 10.1016/J.CELL.2022.11.028. - DOI - PMC - PubMed
-
- Asp M. Developmental heart - filtered and unfiltered count matrices and meta tables. Mendeley Data Version. 2021;2 doi: 10.17632/MBVHHF8M62.2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

