Validation of a semi-quantitative method to assess interstitial lung disease severity and progression in systemic sclerosis by standard and low-dose HRCT scans
- PMID: 40021203
- PMCID: PMC11873339
- DOI: 10.1136/rmdopen-2024-004938
Validation of a semi-quantitative method to assess interstitial lung disease severity and progression in systemic sclerosis by standard and low-dose HRCT scans
Abstract
Background: While the presence of distinct imaging abnormalities by high-resolution CT (HRCT) defines interstitial lung disease (ILD), there is a relative lack of validated methods to quantify these abnormalities in clinical practice, limiting ILD severity and progression assessments. We aimed to validate a semi-quantitative method for lung fibrosis assessment in patients with systemic sclerosis associated ILD (SSc-ILD) by standard and low-dose HRCT, considering lung structure and function as integral components of ILD evaluation.
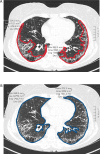
Methods: SSc patients from Oslo and Zurich with HRCT images, pulmonary function tests, including forced vital capacity (FVC), diffusing capacity for carbon monoxide (DLCO) and the 6-minute walk test with oxygen (O2) desaturation were enrolled. We validated the semi-quantitative fibrosis extent method by HRCT using criteria for content and construct validity, discrimination, sensitivity to change and feasibility, as well as inter- and intra-rater variability.
Results: 65 SSc patients from Zurich and 90 from Oslo were included. Significant correlations were observed between the extent of fibrosis on HRCT and FVC (r=-0.517, p<0.001), DLCO (r=-0.400, p<0.001) and O2 desaturation (r=-0.500, p<0.001), indicating content, construct and criterion validity. Discrimination and sensitivity to change assessments showed moderate correlation with DLCO (r=-0.377, p=0.003) but not with FVC or O2 desaturation. Inter- and intra-rater variability demonstrated excellent reliability (κ=0.891 and κ=0.996, respectively), with HRCT quantification averaging 9-15 min, indicating high feasibility.
Conclusion: This study confirms that semi-quantitative fibrosis assessment of HRCT for SSc-ILD meets most validation criteria, supporting its use in clinical practice and showing additive value of structural to functional ILD assessment.
Keywords: Outcome and Process Assessment, Health Care; Pulmonary Fibrosis; Scleroderma, Systemic.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: LT, SJ and TMA report no conflicts. MB reports research funding, consultancy fees and/or speaker fees from GSK, Amgen, Novartis and Vifor. CB reports no conflicts. CC reports consulting fees and speaker fees from GSK, Novartis, Vifor, Boehringer, Astra Zeneca and Sanofi; speaker fees from Daiichi Sankyo; received support for attending meetings from Boehringer and Astra Zeneca. RD reports grants from the Iten-Kohaut Foundation; received support for attending meetings from Otsuka and Amgen; participated on an advisory board for Boehringer Ingelheim. MTD reports grants from Boehringer Ingelheim and Roche; received support for attending meetings and participated on an advisory board for Boehringer Ingelheim. ME received support for attending meetings from Janssen and Astra Zeneca. TF reports speaker fees from Bracco, Bayer and Boehringer; participated on an advisory board for AGFA; holds leadership or fiduciary roles with European Society of Thoracic Imaging and Swiss Society of Radiology. HF reports consulting fees from Bayer; speaker fees from Boehringer Ingelheim. GT and ØMolberg report no conflicts. CM reports speaker fees from MEDtalks Switzerland, Mepha, PlayToKnow AG, Medbase AG, Romanian Society of Rheumatology and Boehringer Ingelheim; support for attending meetings from Boehringer Ingelheim and Roche; participated on an advisory board for Boehringer Ingelheim and Janssen. OMidtvedt reports no conflicts. OD reports grants from Kymera, Mitsubishi Tanabe and Boehringer Ingelheim; consulting fees from 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant Sciences, Amgen, AnaMar, Arxx, Astra Zeneca, Blade Therapeutics, Bayer, Boehringer Ingelheim, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Gossamer, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Orion, Prometheus Biosciences, Redxpharma, Roivant and Topadur; speaker fees from Bayer, Boehringer Ingelheim, Janssen and Medscape; has a patent issued for 'mir-29 for treatment of systemic sclerosis'; hold leadership or fiduciary roles with FOREUM foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Disease), SAMW (Swiss Academy of Medical Sciences) and Hartmann Müller Foundation; and reports other interest with Citus AG. A-MH-V reports grants from BI and Janssen; speakers fees from: Boehringer Ingelheim, Janssen, Medscape, Merck Sharp & Dohme, Novartis and Roche; consultancy fees of: ARXX, Boehringer Ingelheim, Genentech, Janssen, Medscape, Merck Sharp & Dohme, Pliant therapeutics, Roche and Werfen; and holds leadership or fiduciary roles with EULAR, and the Nordic PH vision group.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
