Metabolic dysregulation contributes to the development of dysferlinopathy
- PMID: 40021220
- PMCID: PMC11871293
- DOI: 10.26508/lsa.202402991
Metabolic dysregulation contributes to the development of dysferlinopathy
Abstract
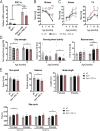
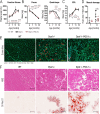
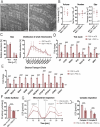
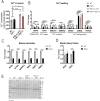
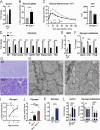
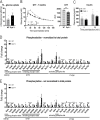
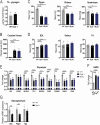
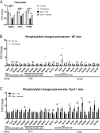
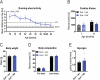
Dysferlin is a transmembrane protein that plays a prominent role in membrane repair of damaged muscle fibers. Accordingly, mutations in the dysferlin gene cause progressive muscular dystrophies, collectively referred to as dysferlinopathies for which no effective treatment exists. Unexpectedly, experimental approaches that successfully restore membrane repair fail to prevent a dystrophic phenotype, suggesting that additional, hitherto unknown dysferlin-dependent functions contribute to the development of the pathology. Our experiments revealed an altered metabolic phenotype in dysferlin-deficient muscles, characterized by (1) mitochondrial abnormalities and elevated death signaling and (2) increased glucose uptake, reduced glycolytic protein levels, and pronounced glycogen accumulation. Strikingly, elevating mitochondrial volume density and muscle glycogen accelerates disease progression; whereas, improvement of mitochondrial function and recruitment of muscle glycogen with exercise ameliorated functional parameters in a mouse model of dysferlinopathy. Collectively, our results not only shed light on a metabolic function of dysferlin but also imply new therapeutic avenues aimed at promoting mitochondrial function and normalizing muscle glycogen to ameliorate dysferlinopathies, complementing efforts that target membrane repair.
© 2025 Furrer et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
