The epidemiology, pathology and pathogenesis of MS: Therapeutic implications
- PMID: 40021419
- PMCID: PMC12418443
- DOI: 10.1016/j.neurot.2025.e00539
The epidemiology, pathology and pathogenesis of MS: Therapeutic implications
Abstract
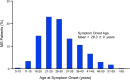
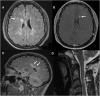
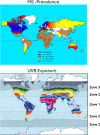
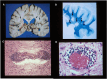
Multiple sclerosis (MS) is a chronic, and potentially disabling, inflammatory disease of the central nervous system (CNS). MS is generally characterized by recurrent, and self-limited, episodes of neurological dysfunction, which occur unpredictably and often result in multifocal tissue injury within the CNS. Currently, women are affected two to three times as often as men although this may not have been the case during earlier Time-Periods. The pathogenesis of MS is known to involve both critical genetic and environmental mechanisms. Nevertheless, in addition to these two mechanisms, disease-pathogenesis also involves a "truly" random event. Indeed, it is this random mechanism, which is responsible for the currently-observed (and increasing) excess of women among patients with MS. This review summarizes the current state of knowledge regarding the pathogenesis of MS (includong its epidemiology, pathology, and genetics) and considers the therapeutic implications that these pathogenetic mechanisms have both for our currently available therapies as well as for the possible therapeutic approaches to the management of this potentially disabling condition in the future.
Keywords: Genetics; Multiple sclerosis epidemiology; Pathogenesis; Pathology; Therapy.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Ruggieri M., Polizzi A., Pavone L., Grimaldi L.M. Multiple sclerosis in children under 6 years of age. Neurology. 1999;53:478–484. - PubMed
-
- Confavreux C., Vukusic S., Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126:770–782. - PubMed
-
- Gadoth N. Multiple sclerosis in children. Brain Dev. 2003;25:229–232. - PubMed
-
- Confavreux C., Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129:606–616. - PubMed
-
- Compston A., Confavreux C., Lassmann H., McDonald I., Miller D., Noseworthy J., et al. fourth ed. Churchill Livingston; London: 2006. McAlpine's multiple sclerosis.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

