Neuron-specific isoform of PGC-1α regulates neuronal metabolism and brain aging
- PMID: 40021651
- PMCID: PMC11871081
- DOI: 10.1038/s41467-025-57363-y
Neuron-specific isoform of PGC-1α regulates neuronal metabolism and brain aging
Abstract
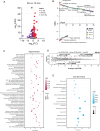
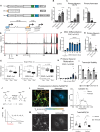
The brain is a high-energy tissue, and although aging is associated with dysfunctional inflammatory and neuron-specific functional pathways, a direct connection to metabolism is not established. Here, we show that isoforms of mitochondrial regulator PGC-1α are driven from distinct brain cell-type specific promotors, repressed with aging, and integral in coordinating metabolism and growth signaling. Transcriptional and proteomic profiles of cortex from male adult, middle age, and advanced age mice reveal an aging metabolic signature linked to PGC-1α. In primary culture, a neuron-exclusive promoter produces the functionally dominant isoform of PGC-1α. Using growth repression as a challenge, we find that PGC-1α is regulated downstream of GSK3β independently across promoters. Broad cellular metabolic consequences of growth inhibition observed in vitro are mirrored in vivo, including activation of PGC-1α directed programs and suppression of aging pathways. These data place PGC-1α centrally in a growth and metabolism network directly relevant to brain aging.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no conflict of interest.
Figures





Update of
-
Mitochondrial regulator PGC-1a in neuronal metabolism and brain aging.bioRxiv [Preprint]. 2023 Sep 29:2023.09.29.559526. doi: 10.1101/2023.09.29.559526. bioRxiv. 2023. Update in: Nat Commun. 2025 Feb 28;16(1):2053. doi: 10.1038/s41467-025-57363-y. PMID: 37808866 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

