Ligand-receptor interactions combined with histopathology for improved prognostic modeling in HPV-negative head and neck squamous cell carcinoma
- PMID: 40021759
- PMCID: PMC11871237
- DOI: 10.1038/s41698-025-00844-6
Ligand-receptor interactions combined with histopathology for improved prognostic modeling in HPV-negative head and neck squamous cell carcinoma
Abstract
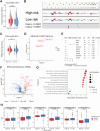
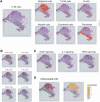
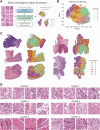
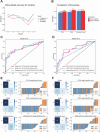
Head and neck squamous cell carcinoma (HNSC) is a prevalent malignancy, with HPV-negative tumors exhibiting aggressive behavior and poor prognosis. Understanding the intricate interactions within the tumor microenvironment (TME) is crucial for improving prognostic models and identifying therapeutic targets. Using BulkSignalR, we identified ligand-receptor interactions in HPV-negative TCGA-HNSC cohort (n = 395). A prognostic model incorporating 14 ligand-receptor pairs was developed using random forest survival analysis and LASSO-penalized Cox regression based on overall survival and progression-free interval of HPV-negative tumors from TCGA-HNSC. Multi-omics analysis revealed distinct molecular features between risk groups, including differences in extracellular matrix remodeling, angiogenesis, immune infiltration, and APOBEC enzyme activity. Deep learning-based tissue morphology analysis on HE-stained whole slide images further improved risk stratification, with region selection via Silicon enhancing accuracy. The integration of routine histopathology with deep learning and multi-omics data offers a clinically accessible tool for precise risk stratification, facilitating personalized treatment strategies in HPV-negative HNSC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68, 394–424 (2018). - PubMed
-
- Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin.71, 209–249 (2021). - PubMed
-
- Taberna, M. et al. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol.28, 2386–2398 (2017). - PubMed
LinkOut - more resources
Full Text Sources

