FGF21, a modulator of astrocyte reactivity, protects against ischemic brain injury through anti-inflammatory and neurotrophic pathways
- PMID: 40021824
- PMCID: PMC12205086
- DOI: 10.1038/s41401-024-01462-x
FGF21, a modulator of astrocyte reactivity, protects against ischemic brain injury through anti-inflammatory and neurotrophic pathways
Abstract
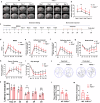
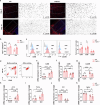
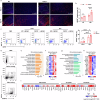
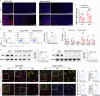
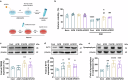
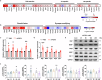
Ischemic stroke is a frequent cause of mortality and disability, and astrocyte reactivity is closely associated with injury outcomes. Fibroblast growth factor 21 (FGF21), an endogenous regulator, has been shown to perform pleiotropic functions in central nervous system (CNS) disorders. However, studies on neurological diseases have paid little attention to the effects and detailed mechanisms of FGF21 in astrocytes. Here, we found elevated serum levels of FGF21 in stroke patients and transient middle cerebral artery occlusion (tMCAO) mice. In the peri-infarct cortex, microglia and astrocytes serve as sources of FGF21 in addition to neurons. MRI and neurobehavioral assessments of wild-type (WT) and FGF21-/- tMCAO model mice revealed a deteriorated consequence of the loss of FGF21, with exacerbated brain infarction and neurological deficits. Additionally, combined with the pharmacological treatment of WT mice with recombinant human FGF21 (rhFGF21) after tMCAO, FGF21 was identified to suppress astrocytic activation and astrocyte-mediated inflammatory responses after brain ischemia and participated in controlling the infiltration of peripheral inflammatory cells (including macrophages, neutrophils, monocytes, and T cells) by modulating chemokines expression (such as Ccl3, Cxcl1, and Cxcl2) in astrocytes. Furthermore, rhFGF21 was shown to boost the production of neurotrophic factors (BDNF and NGF) in astrocytes, and by which rescued neuronal survival and promoted synaptic protein expression (postsynaptic density protein-95 (PSD-95), synaptotagmin 1 (SYT1), and synaptophysin) in neurons after ischemic injury. Overall, our findings implicate that FGF21 acts as a suppressor of astrocyte activation, and exerts anti-inflammatory and neurotrophic effects after ischemic brain injury through its action on astrocytes, offering an alternative therapeutic target.
Keywords: FGF21; astrocyte reactivity; leukocyte infiltration; neuroinflammation; transient middle cerebral artery occlusion (tMCAO).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

